Group coles
Project 1: Fundamental Chemistry of the heavier Pnictogens (Sb and Bi)
We have an interest in developing the fundamental chemistry of the metallic elements. Recently we have concentrated these efforts on the heavier elements of group 15 elements (the Pnictogens), focusing on Antimony (Sb) and Bismuth (Bi). We employ chelating silyl-diamide ligands to support the metal, which enables us to explore the reactivity of the resultant compounds towards various substrates. We specifically target low-coordinate species that allow small molecules to approach the metal centre and undergo chemical transformations.
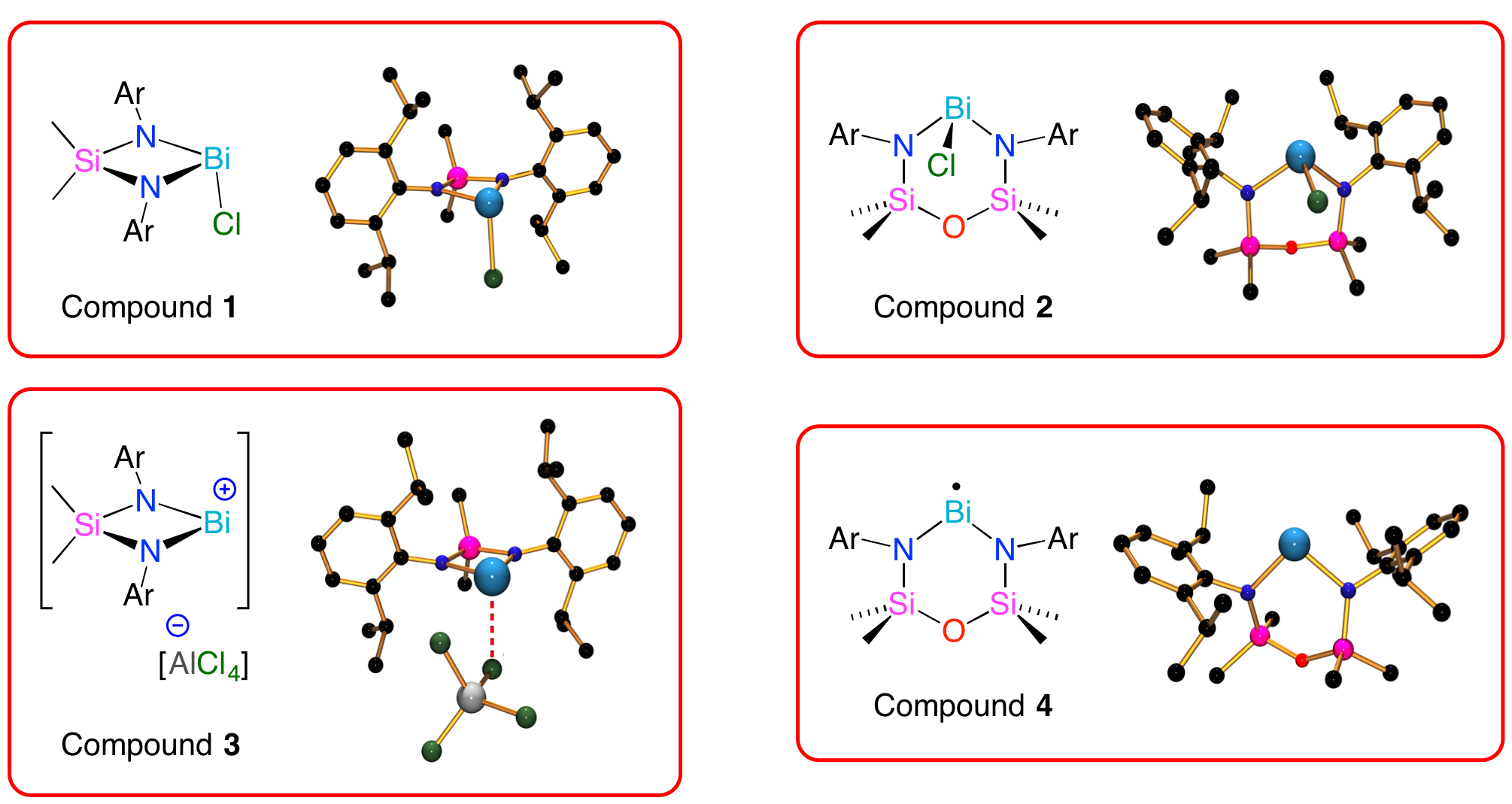
The two ligand systems that we have applied in this area differ in the size of the metallacycle (1 = 4-membered metallacycle; 2 = 6-membered metallacycle), and hence offer a degree of control over the environment at the metal. This has allowed us to synthesize low-coordinate cations (3),[1] and recently we have isolated the first monomeric bismuth(II) compound (4), which exists as a radical in the solid-state.[2] We are currently exploring the reactivity of compound 4 towards a number of organic and inorganic substrates.
Project 2: Novel super-bases
A synthetic project that involves mainly organic chemistry involves the development of new super-bases, defined in this project as a compound with a basicity higher than 'proton-sponge' (i.e. pKa(MeCN) > 18.6). We achieve this by combining multiple guanidine functionalities on different molecular scaffolds, aiming to promote stabilization of the conjugate acid through the formation of intramolecular hydrogen-bonds.
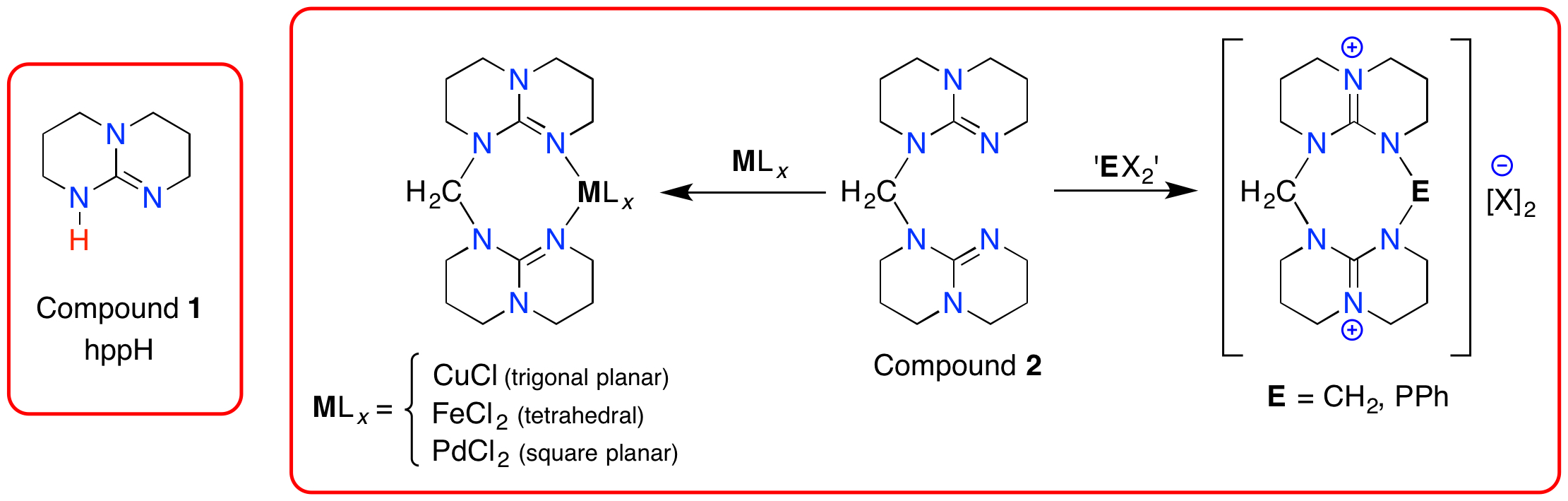
Our work has concentrated on the bicyclic guanidine 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine framework (1, abbreviated as hppH), which has an inherent pKa of 26.0. We have shown that the pKa can be increased by three orders of magnitude when two groups are joined by a simple methylene-linker (2, pKa = 29.0).[3] Compound, 2 also behaves as a neutral chelating ligand,[4] and promotes nucleophilic behavior from the guanidine groups affording unusual heterocycles.[5]
Current work involves expanding this concept to include different linking groups able to support multiple guanidine groups. We have also included additional functionality in the bridging group to enhance the basicity through formation of intra-molecular hydrogen bonds (e.g. pyridyl, phosphorus-based groups).
Spin-off projects
2a Carbon-Dioxide Activation
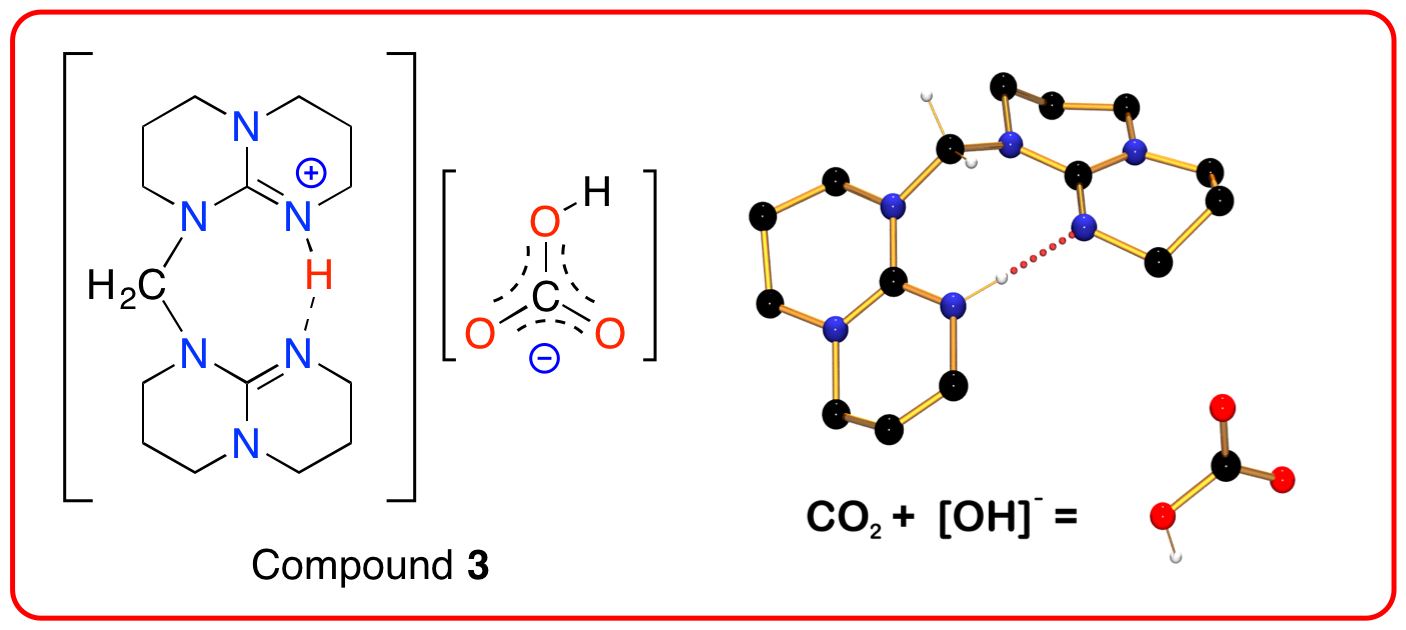
Compound 2 is able to activate atmospheric carbon-dioxide in the presence of water to afford the bicarbonate salt, [H2C{hpp}{hppH}][HCO3] (3). We are investigating the generality of this reaction with other super-bases synthesized in our laboratory.
2b Organometallic Chemistry
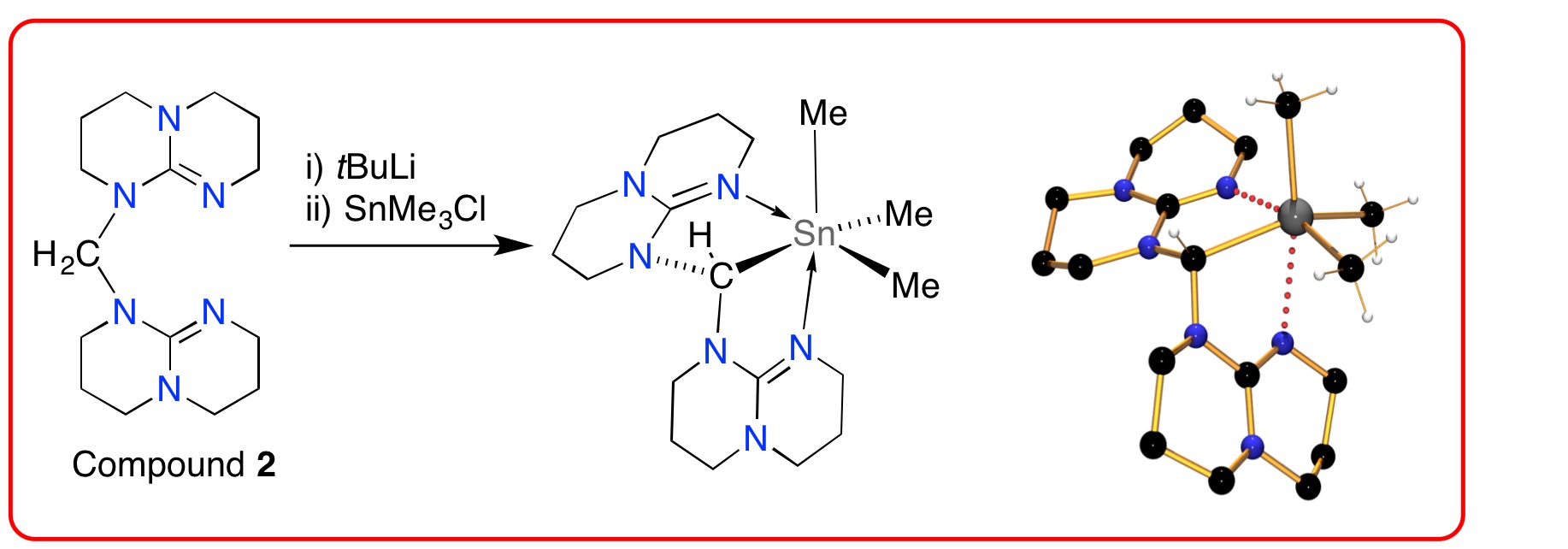
Deprotonation of the methylene-linker group of compound 2 affords the corresponding carbanion, which has been used as a pincer-type ligand in coordination chemistry (e.g. 4).[6]
Project 3: Ferrocenyl-Amidinates: Molecular switches and functional materials
An amidine is a nitrogen-based organic fragment consisting of a central sp2-carbon bonded to an imine- and an aminefunctional group (A). It can be protonated at the imine nitrogen atom to afford the corresponding amidinium cation (B), that is able to form Charge-Assisted Hydrogen-Bonds (CAHBs) with a suitable counter-anion (C).
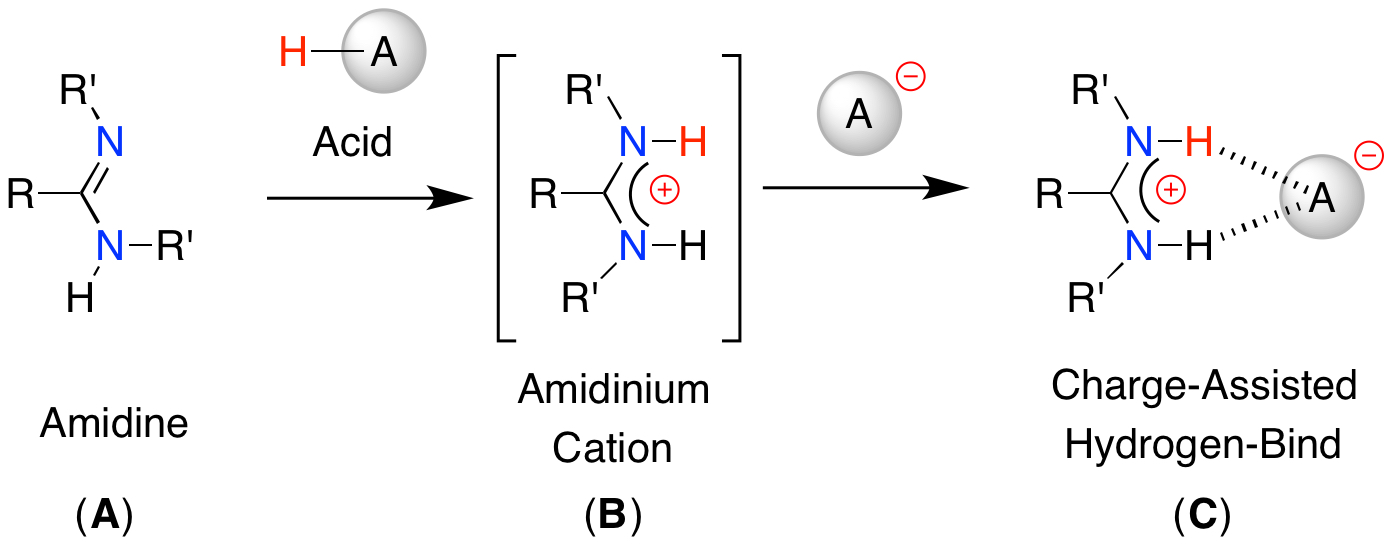
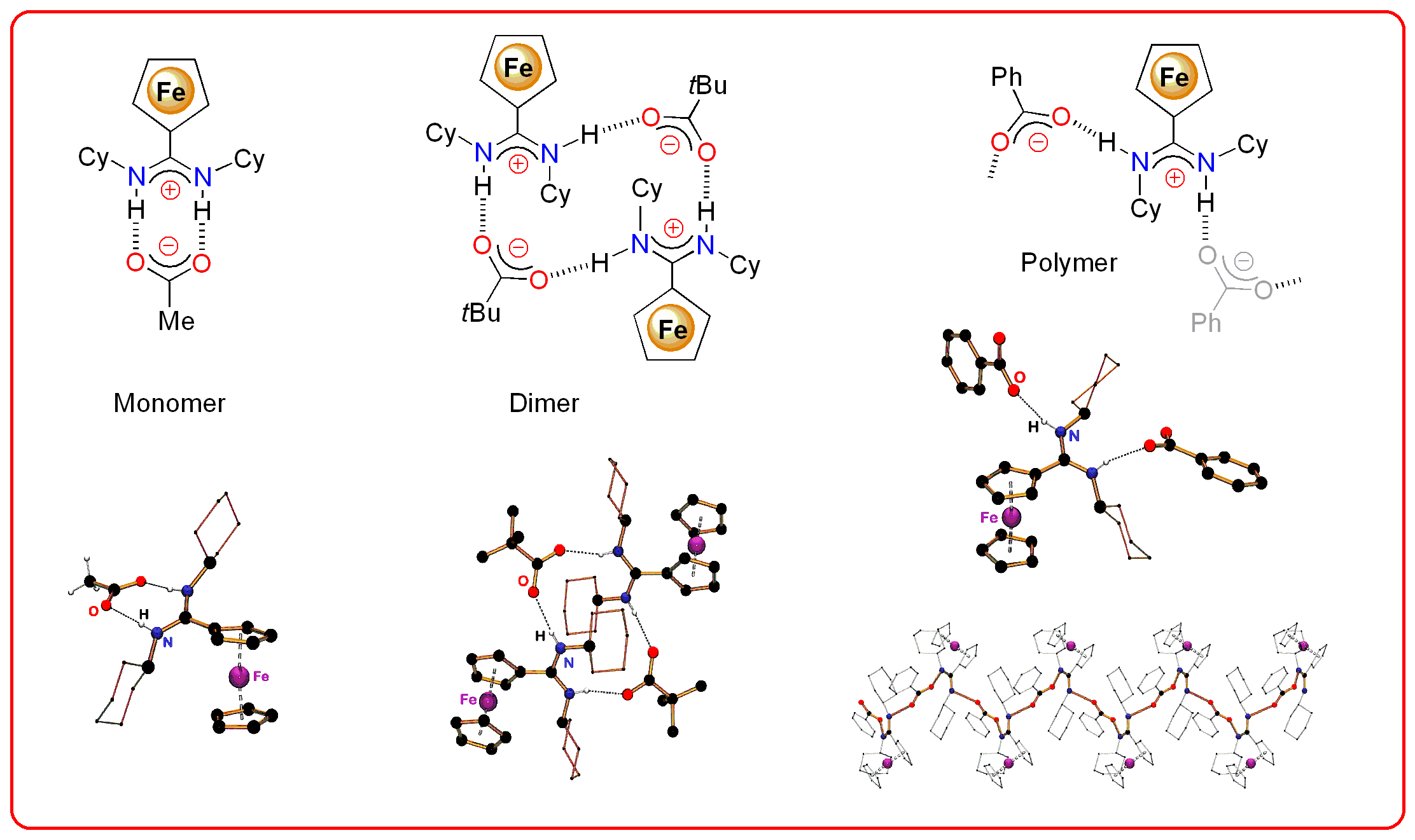
We have used this approach to construct complex molecular architectures that extend in 1- or 2-dimensions. We have selected a system that incorporates a ferrocenyl-group that can act as a molecular reporter group using different analytical techniques (e.g. electrochemistry, UV/vis spectroscopy). To date we have concentrated on CAHBs with carboxylate anions and have observed monomers, dimers and polymeric systems in the solid-state, depending on the carboxylate substituent.
References
[1] Ryan J. Schwamm, Benjamin M. Day, Martyn P. Coles and Christopher M. Fitchett, "Low-Coordinate Bismuth Cations", Inorg. Chem. 2014, 53, 3778-3787.
[2] Ryan J. Schwamm, Jeffrey R. Harmer, Matthias Lein. Christopher M. Fitchett, Simon Granville and Martyn P. Coles, "Isolation and Characterization of a Bismuth(II) Radical", Angew. Chem. Int. Ed. 2015, 54, 10630-10633.
[3] Martyn P. Coles, Pedro J. Aragón-Sáez, Sarah H. Oakley, Peter B. Hitchcock, Matthew G. Davidson, Zvonimir B. Maksić, Robert Vianello, Ivo Leito, Ivari Kaljurand and David C. Apperley, "Superbasicity of a Bis-guanidino Compound with a Flexible Linker: A Theoretical and Experimental Study", J. Am. Chem. Soc., 2009, 131, 16858-16868.
[4] (a) Sarah H. Oakley, Martyn P. Coles and Peter B. Hitchcock, "Multiple Coordination Geometries Supported by Methylene-Linked Guanidines", Inorg. Chem. 2004, 43, 7564-7566. (b) Majid S. Khalaf, Sarah H. Oakley, Martyn P. Coles and Peter B. Hitchcock, "Coordination of Neutral, Methylene Bridged Bis-guanidyls at Palladium", Dalton Trans. 2010, 39, 1635-1642.
[5] (a) Martyn P. Coles, Steven F. Lee, Sarah H. Oakley, Guillermina Estiu and Peter B. Hitchcock, "Nucleophilic Activity of a Linked Bis{guanidine} Leading to Formation of a Dicationic C4N4-Heterocycle", Org. Biomol. Chem. 2007, 5, 3909-3911. (b) Martyn P. Coles and Peter B. Hitchcock, "A Lewis-Basic, Dionio-Substituted Phosphane", Chem. Commun. 2007, 5229-5231.
[6] Martyn P. Coles, Majid S. Khalaf and Peter B. Hitchcock, "A New Aliphatic N,C,N'-pincer Ligand with Pendant Guanidine Groups", Inorg. Chim. Acta 2014, 422, 228-234 (Invited Contribution – T. Don Tilley special issue).
