News Archives
Immunologists receive over $2m for MS and flu vaccine projects
Professor Anne La Flamme and Dr Lisa Connor given nearly $1.2m each in grants from the Health Research Council of New Zealand.
Published 28 July 2021
Two Te Herenga Waka—Victoria University of Wellington immunologists are among the successful recipients of funding from the Health Research Council (HRC) of New Zealand announced yesterday.
Professor Anne La Flamme and Dr Lisa Connor from Te Kura Mātauranaga Koiora—School of Biological Sciences have received nearly $1.2 million each for their research over the next three years, as part of the HRC’s 2021 Project Grants round.
Professor La Flamme’s research will look at how brain inflammation is prevented using a novel chemical compound, designed by Dr Olga Zubkova from the University’s Te kāuru—Ferrier Research Institute, that inhibits the harm done by the enzyme heparanase. Her team’s work could ultimately lead to the development of therapeutics to prevent damage caused by immune cells in diseases such as multiple sclerosis.
“We have two key aims, one basic science aim and a second practical aim,” says Professor La Flamme.
“First, we want to understand how immune cells enter the brain during health and during neurological diseases such as multiple sclerosis, meningitis, schizophrenia, Alzheimer’s, and traumatic brain injury.
“In particular we will investigate the specific molecules in the barriers that divide the brain from the rest of the body. These barriers control the entrance of immune cells and if this control is compromised damaging brain inflammation can result.
"The second aim is to develop a therapy that targets these specific structures so we can restore normal control of immune cell migration through these barriers. Using novel chemistry, we have created a compound we can use to help develop innovative targeted therapeutics in the future.”
Dr Connor and her team are looking at how lung resident immune cells can be used to create mucosal vaccines for respiratory pathogens such as the influenza virus, which kills about 500 New Zealanders a year.
These vaccines can be delivered orally or intranasally to induce mucosal immunity, which provides a first line of defence, working quickly and potentially preventing diseases spreading to other parts of the body.
The team’s work could ultimately influence the design of the next generation of mucosal vaccines for influenza.
Dr Connor says protection against respiratory pathogens is initiated by the body’s mucosal immune system but most licensed vaccines are administered by an injection and do not induce fast-acting immunity at the site of infection.
Rigorous safety requirements mean new vaccines often require inclusion of an adjuvant, which enhances the body’s immune response.
“This poses a major barrier for mucosal delivery, as most adjuvant formulations are not suitable. Our research focuses on a novel class of adjuvants that harness resident innate-like T cells. Importantly, we have generated evidence to show that when these adjuvants are co-administered with an antigen they induce potent antibody responses.”
The goal of the project is to identify the key immune cells and molecular events involved in driving the immune response, which in turn will inform the formulation and structural design of an optimal mucosal vaccine for influenza.
Dr Connor says using Aotearoa New Zealand-based biotech companies to manufacture vaccines developed as a result of the project could result in a significant economic return and improve health outcomes for New Zealanders.
The development of an effective mucosal adjuvant could also provide a significant advantage for future COVID vaccines, she says.
The cross-disciplinary team involved in the project includes Professor Ian Hermans, deputy director of the Malaghan Institute of Medical Research, along with Professor Gavin Painter and Dr Benji Compton from the Ferrier Research Institute.
Professor La Flamme’s ground-breaking preliminary work was supported by charitable donations from the Great New Zealand Trek and individual donors to the Malaghan Institute’s multiple sclerosis research programme. Those donations also enabled the establishment of an international team for the project. As well as Professor La Flamme and Dr Zubkova, the team includes Dr Gill Webster from ImmunoStrategy and Professor Christopher Parish and Dr Anne Bruestle from the John Curtin School of Medical Research at Australian Nacional University.
First NZ study to diagnostically validate COVID-19 saliva testing
The more tools we have in our toolbox to fight COVID-19 and keep the country safe, the better, says study leader Associate Professor Janet Pitman.
Published 25 June 2021
Scientists at Te Herenga Waka—Victoria University of Wellington have used real-world samples to confirm the 98.7 percent accuracy of a saliva test for COVID-19. This is the first, and currently the only, COVID-19 saliva test to be diagnostically validated in Aotearoa New Zealand.
Associate Professor Janet Pitman from the University’s Te Kura Mātauranga Koiora—School of Biological Sciences led the study. “Despite a view that saliva tests aren’t as accurate as the standard nasopharyngeal test, our research shows this one is,” she says. A paper describing the study has been prepared and submitted for publication in a medical journal.
Initial testing was carried out on artificially infected saliva, where heat-inactivated SARS-CoV-2 virus, which causes COVID-19, was added to saliva in the lab. The now-completed diagnostic validation involved testing samples from actual positive COVID-19 patients.
Associate Professor Pitman’s team tested paired samples, sent from the United States, from 152 people. The paired samples were a sample of saliva and a sample from a nasal swab taken at the same time. Thirty-four of these people were positive for COVID-19.
In all but two instances, the results matched—in one instance the saliva sample tested positive when the nasopharyngeal sample didn’t, and in the other the reverse occurred. The discordance between the two samples is likely not due to a failing of either test. It is more likely due to differences in the timing of the virus’s presence during the early stages, and disappearance during the late stages, of the disease at the two biological sites (salivary glands and the nasopharyngeal region). Salivary glands contain large numbers of cells that replicate the virus and are then shed into the saliva, making saliva a great indicator of live virus production.
The researchers’ results showed the test is highly sensitive. “An infectious person has about 1,000 viral copies of SARS-CoV-2 per microlitre of saliva, equivalent to one thousandth of a millilitre,” says Associate Professor Pitman. “Our test can measure one copy in this volume. It is as sensitive as the standard nasopharyngeal test. We found this test is sensitive enough to measure asymptomatic people well before they become infectious.”
Associate Professor Pitman says there are many advantages to this method. “The great thing about saliva testing is not only can you use it for detection but also as an ongoing surveillance method to keep an eye on what’s in the community. You can test more regularly as it’s non-invasive.
“I’m not sure how long the public is going to accept the nasopharyngeal ‘brain scrape’ test. With our test, you just let the saliva pool in your mouth, then drool into a spoon, without creating bubbles, and tip it into a tube. However, people must not eat or drink for one hour before providing the saliva sample to ensure a good quality sample.”
There are also other advantages to saliva tests, she says—for example, they don’t require health care professionals to take the sample.
Associate Professor Pitman stresses that COVID tests are going to be part of our lives for years to come.
“This virus isn’t going away, and eventually we’re going to open our borders. What we need is a suite of highly accurate, diagnostically validated saliva tests for SARS-CoV-2. And for that to happen, the information surrounding saliva tests should be transparent and publicly available. We’re going to need these tests in our workplaces to give people peace of mind at work, at our borders to enable people to travel, and within our communities to gauge any outbreaks. The more tools we have in our toolbox to fight this and keep New Zealand safe, the better.”
The saliva test was developed by the University of Illinois in the US and is authorised for emergency use by the US Food and Drug Administration. The test is provided in New Zealand by Rako Science, which asked Associate Professor Pitman’s team to validate the test in New Zealand, for rollout here through the molecular diagnostic lab iGENZ. It is currently being used at Auckland airport, Air New Zealand and other large workplaces and will also service the New Zealand Olympic team.
Study shows a short time to save coral reefs
University marine biologist Dr Christopher Cornwall has some good news to offer amid a grim outlook because of the scale of carbon dioxide emissions.
Published 11 May 2021

The window of opportunity to save the world’s coral reefs is still open but time is running out, new research shows.
An international study jointly led by Te Herenga Waka—Victoria University of Wellington marine biologist Dr Christopher Cornwall has calculated how coral reefs are likely to react to ocean acidification and warming under three different climate change carbon dioxide scenarios—low, medium, and worst-case.
The study, just published in the journal PNAS, has some good news to offer amid a grim outlook.
Dr Cornwall, a Rutherford Discovery Fellow in the School of Biological Sciences, who earlier this year won a $200,000 Prime Minister’s MacDiarmid Emerging Scientist Prize, says if the world can reduce carbon dioxide emissions drastically coral reef growth will be reduced “but many reefs will still be able to grow.
“Some of them will even keep pace with sea-level rise. Even if we fail with those reductions but do keep within the intermediate emissions scenario, some coral reefs will still keep growing for a short while, but by the end of the century they will all be eroding.
“If we hit the worst-case scenario, very shortly all coral reefs will be eroding.”
The research by academics in New Zealand, Australia, the United States, France, the Netherlands, and the United Kingdom breaks new ground.
Jointly led by Dr Cornwall and French colleague Dr Steeve Comeau, the interdisciplinary group of scientists initially formed in 2016 as a working group led by the Australian Research Council’s Centre of Excellence for Coral Reef Studies.
Although there has been research investigating the impact of climate change on individual corals and coralline algae, the new study gives broader projections of ocean warming and acidification, and their interaction, on the net carbonate production of coral reefs.
“We have known for more than a decade ocean acidification will affect the ability of calcifying coral reef taxa to form their calcium carbonate skeletons, a process called ‘calcification’,” says Dr Cornwall.
“Also, we have known ocean warming brings increasing frequencies of marine heatwaves that cause coral mass bleaching, but that this warming itself also reduces calcification once it gets above a certain threshold.
“Although there was a wealth of information known about how certain organisms would fare under climate change, coral reef growth is not just the product of coral calcification and survival. Calcifying red algae, known as coralline algae, glue these reefs together and even form their own reefs in certain places in the world’s oceans.
“While corals are highly susceptible to ocean warming, coralline algae are more vulnerable to ocean acidification. Coral reef growth is also dictated by the removal of this calcium carbonate through either bioerosion—living organisms eating the reef—or the dissolution of sediments that help fill in the cracks between larger pieces of calcium carbonate.
“Both processes are likely to accelerate under ocean acidification and warming. However, no one study had put these processes together quantitatively previously.”
There are thousands of coral reefs around the world, each comprising different proportions of corals and coralline algae, with different types of bioeroders, such as parrotfish, sea urchins, and cyanobacteria, and different rates of sediment production.
The study used data on net calcification, bioerosion, and sediment dissolution rates measured or collated from 233 locations on 183 distinct reefs, 49 percent of them in the Atlantic Ocean, 39 percent in the Indian Ocean, and 11 percent in the Pacific Ocean.
This was then modelled against three Intergovernmental Panel on Climate Change emissions scenarios for low-, medium-, and high-impact outcomes on ocean warming and acidification for 2050 and 2100.
Dr Cornwall says their projections show most coral reefs will be unable to maintain growth from carbonate production by the end of the century under the medium- and high-impact scenarios. Even under the low-impact scenario, reefs will suffer severely reduced accretion rates.
“We forecast mean global reef net-carbonate production under these three pathways will decline by 76 percent, 149 percent, and 156 percent respectively by 2100.
“While 63 percent of reefs are projected to continue to accrete by 2100 under the low-impact pathway, 94 percent will be eroding by 2050 under the worst-case scenario, and no reefs will continue to accrete at rates matching projected sea-level rise under the medium- and high-impact scenarios by 2100.”
Drastic reductions in carbon dioxide emissions are now needed to give coral reefs the best chance of continuing to accrete in a future ocean, says Dr Cornwall.
“We are already observing global shifts in coral assemblages and severely reduced coral cover due to mass bleaching events. It is very unlikely corals will suddenly gain the heat tolerance required to resist these events as they become more frequent and intense.
“Our only hope for these reefs now is converting to alternatives to fossil fuels as soon as possible.”
Dr Christopher Cornwall has co-written an article about the study for The Conversation, which can be republished for free under Creative Commons: https://theconversation.com/rising-co2-emissions-will-halt-coral-reef-growth-without-action-160251
Kauri and the community
A University-led education and outreach project called Te Kura O Te Kauri has spent the last month aiming to inspire over 1000 students, teachers, family and community members to become guardians of their kauri forests.
22 November 2019

This project, led by Dr Monica Gerth, senior lecturer in Microbiology, worked to introduce schoolchildren to the science and mātauranga behind forest health, and to spread awareness about stopping the spread of kauri dieback disease. It was hosted in a travelling classroom, allowing Dr Gerth and her team to take the project to several school and community sites around the Northland region.
Te Kura O Te Kauri incorporated different modules covering science, art, and mātauranga.
“Each stop also included aspects of local culture and mātauranga that was delivered by our local engagement leaders and their teams. This helped us build a special connection with the communities we visited,” Dr Gerth says.
One of the most popular modules was a virtual reality (VR) experience developed by Dr Wayne Patrick, Associate Professor of Biochemistry at Victoria University of Wellington, in collaboration with digital designer Jeff Jones, sound engineer Jimi Wilson, and Master’s student Te Amohaere Ngata-Aerengamate. Using the VR technology, students move from the crown of a kauri tree down into the soil at the microscopic scale.
For Dr Patrick, it was rewarding to see the VR in action. “Visitors engaged with the VR in so many different ways, from screaming with excitement, to reaching out to grab invisible microbes...it shows how immersive the VR is and how powerful it is as an unforgettable learning experience.”
Dr Gerth and her team were especially excited to take Te Kura O Te Kauri to Northland because of the shortage of science education and equipment available in rural regions.
“This classroom gives tamariki access to cutting edge scientific equipment, knowledge and resources—and the feedback has been overwhelmingly positive,” says Abigail Sucsy, Te Kura O Te Kauri coordinator. “It was awesome to see how engaged and excited the students were—they didn’t want to leave!”
Their first tour of Northland finished in early November, but Dr Gerth and her team are not planning to stop now.
“We want to continue this mahi,” says Abigail. “Our current funding ends next month, but we hope to raise enough money to visit more schools and communities. There has already been a huge amount of interest from communities we weren’t able to visit on our first tour.”
The team is following up with teachers, and also sending out study modules and equipment to be used in classrooms. While their main focus is Northland, they have run pop-up sessions in the School of Biological Sciences at the University, and are also looking in to running other pop-up events in the Wellington area.
Te Kura O Te Kauri was funded by an MBIE Unlocking Curious Minds grant, with additional support from the University. To find out more about Te Kura O Te Kauri, or to request a visit for your school or community, visit their Facebook page @kauri.classroom or their website at www.tekuraotekauri.nz.
New study sheds light on effectiveness of cholesterol medication in individuals
A study by a team of Victoria University of Wellington scientists spotlights the role of gene networks in how people respond to one of the world’s most prescribed medications.
4 October 2019

The research team investigated the genetic network response to cholesterol-lowering drugs called statins, a medication prescribed to about 30 million people worldwide. The researchers say it is a significant step towards more targeted, personalised medication.
The work was begun by Dr Bede Busby as a PhD student at the University, working with chemical geneticists Professor Paul Atkinson and Dr Andrew Munkacsi (pictured) from the University’s School of Biological Sciences and Centre for Biodiscovery. It appears in the prestigious scientific journal npj Systems Biology and Applications, published by Nature Research.
“Statins work and deservedly have a good reputation. But 15 percent of patients suffer painful side effects and 50 percent have inadequate response,” says Professor Atkinson. “So what we’ve found out about how statins work can lead to modifying statins to make them more precise, based on personal differences in genetic interaction networks. The aim is to make them effective for people they don’t currently work for (these people are resistant to statins) and this requires understanding complex traits, that is traits that involve many genes, as is the case with all drug responses.”
“To explain a complex trait, it was previously thought that all you had to do was add up the contributing genes—tallness, for example, would be the sum of more than 200 genes,” he says. “But what we’ve shown is that the synergistic interactions between each of the genes turn out to be just as important. The synergies form gene networks and these differ in individuals, so you have to look at the gene network variation of individuals to get a complete picture of how traits are specified by genes and inherited.”
The study of complex traits needs simplifying short cuts so the researchers used baker’s yeast, which is a widely used and a very productive model to study human genetics and how therapeutic drugs work.
“We can do things with yeast that you can’t yet easily do with human cells,” explains Dr Munkacsi. “If you want to know how 6000 genes work synergistically together, you study all pairwise combinations of genes—this is classic methodology in yeast genetics that we adapted to study different genetic backgrounds and it is not yet adapted to study the 21,000 human genes.”
Dr Munkacsi says the research specifically used yeast strains that were resistant to statins. “We did experiments in the resistant yeast strains and worked out the biology of those interactions—that means we have a sense of what processes are involved in that resistance.”
“We integrated advanced biology experimentation, mathematics, statistics, network medicine and adapted social network analysis for complex genetic data in a new approach to looking at drug response. We’re continuing to use this methodology to study other drugs and diseases,” Professor Atkinson says.
“We’ve opened a box that hasn’t really been considered before—it’s experimentally difficult to systematically consider both genetic backgrounds and genetic synergistic interactions so pharmaceutical companies haven’t done it in their drug discovery process,” explains Dr Munkacsi. “But we’ve shown that yes, you should consider both of these as part of drug discovery—used early in the process it could save companies millions of dollars if it identifies undesirable responses.”
All nine co-authors of the paper are currently or formerly based at the University, with Dr Busby now at the European Molecular Biology Laboratory in Heidelberg. “Typically publishing papers requires an international effort, but this one is all New Zealand—and impressively the majority are postgraduate students in our Chemical Genetics Laboratory at Victoria University of Wellington,” says Dr Munkacsi.
“It’s very satisfying to get this research out there, and like all significant research it’s not the end of the road—it opens doors,” says Professor Atkinson. “Our work demonstrates principles that were not necessarily understood before, that also can be applied more widely—to other drugs and diseases, for example. We haven’t discovered a magic bullet but we have discovered some good science.”
Solving a hidden threat to New Zealand’s meat and dairy industry
Beef and lamb exports are one of New Zealand’s major industries, potentially exceeding $3 billion for the first time this year. But a high prevalence of veterinary pathogens causes high rates of animal death, suffering, and decreased production, and diseases like pneumonia in sheep and mastitis in cows lack effective vaccines.
5 September 2019
Associate Professors Bridget Stocker and Mattie Timmer from Victoria University of Wellington are working with AgResearch to help address this problem, developing vaccines to help prevent ovine pneumonia, with promising early results.
This is the next step in an ongoing project for the University researchers, who have spent the past few years developing a new class of vaccine adjuvant—which is an additive to a vaccine that improves the host’s immune response and increases vaccine efficacy. During the development of this adjuvant class, the researchers, along with their PhD student Amy Foster, worked with Professor Sho Yamasaki from Japan, one of the world’s foremost experts in immunology.
“To have an effective vaccine, you need the right adjuvant for the right pathogen,” Associate Professor Stocker says. “There is a gap in the market for adjuvants that elicit a strong cellular immune response in addition to an antibody-mediated response. This is a need we are addressing.”
The adjuvant created at Victoria University of Wellington activates a specific immune pathway. This pathway is related to a number of human diseases, such as meningitis and tuberculosis, but it is also related to the pathogens that cause ovine pneumonia.
With the help of Viclink, the University’s commercialisation arm, and funding from the Ministry of Business, Innovation, and Employment, Associate Professors Stocker and Timmer connected with Drs Neil Wedlock, Natalie Parlane and Axel Heiser from AgResearch, experts in animal vaccines.
“We hadn’t initially considered our adjuvant in relation to animal vaccines, but Viclink suggested this as a possible commercialisation pathway,” Associate Professor Stocker says. “So far our early trials show a lot of promise, and we’re very excited about the next steps.”
“We spend a long time undertaking basic research to understand how particular classes of molecules interact with the immune system. It’s great to be able to take this knowledge from an academic setting to one that could help solve a major issue in the New Zealand farming industry.”
So far, the research team have worked to refine their adjuvant in the laboratory and develop a vaccine for use in sheep. They have completed the first phase of testing and will enter the second phase over the coming months.
“In the first proof-of-concept trial the adjuvant performed as good as, if not better than, currently available adjuvants,” Associate Professor Timmer says. “It is still early days, but it bodes well for future testing.
“Science is never straightforward—if it were there would be no problems left to solve—but we are quietly optimistic, and early indicators suggest we are heading in the right direction.”
Jeremy Jones, Senior Commercialisation Manager at Viclink, says he is very excited to be working with the team to progress their technology towards market.
“Each set of results we get makes us more excited about this project,” Jeremy says. “The team they have assembled makes our job much easier, as we have all of the expertise we need to generate a very strong data package, and the involvement of the team at AgResearch has accelerated this project, allowing us to gather data in a large animal to bolster the laboratory work done here at the University.
“On a recent trip to the US to engage with the animal health market it was clear that there is an imperative from these companies to find technologies that enabled the reduction or elimination of antibiotics from the food-chain. An effective vaccination program and use of immunostimulants such as these developed by Associate Professors Stocker and Timmer and their team are the best line of defence for the industry."
Researchers make potential breakthrough in cancer drug development
A Victoria University of Wellington research team has developed an exciting new lead in the search for cancer treatments, creating alternative versions in the laboratory of a rare natural compound that targets some types of cancer.
21 August 2019
The research team, led by Dr Joanne Harvey from the School of Chemical and Physical Sciences, successfully created several synthetic alternatives to the compound, TAN-2483B, which is found in some fungi. Previous research has shown that this compound may be effective against the development of some types of cancer and can also help with bone degenerative diseases like osteoporosis, but researchers haven’t been able to find or create it in big enough quantities for it to be useful in drug development, Dr Harvey says.
“TAN-2483B has previously only been isolated in small quantities or as mixtures, so it’s very exciting that we’ve been able to create synthetic alternatives in larger quantities in the lab,” Dr Harvey says.
Now they’ve created the alternative compounds Dr Harvey and her team plan to recreate the natural compound in the laboratory as well, which will mean that the therapeutic potential of TAN-2483B can be fully explored.
“Our alternatives and the original compound target different cancer enzymes, so if we can create all of them in good quantities in the lab we will have even more avenues for cancer drug development,” Dr Harvey says.
As well as creating larger quantities in the laboratory, Dr Harvey and her team have been working to make the production process affordable and more accessible. They used a cheap and readily available sugar as the main building block of the alternatives they have developed and are investigating how to make the rest of the production process more efficient as well, Dr Harvey says.
“If we can cheaply and easily produce large quantities of these compounds, it will enable us to perform the thorough tests needed to take them to the next stages of cancer drug development,” Dr Harvey says.
This work was published in Chemistry—An Asian Journal.
Mātauranga Māori could stop kauri dieback in its tracks
Research led by Victoria University of Wellington’s Dr Monica Gerth in collaboration with iwi has discovered molecules from New Zealand native plants could hold the solution to kauri dieback.
20 August 2019
“Our research has discovered that some compounds found in kānuka cause an immediate loss of motility, or movement, of the infectious spores of the microbe that causes kauri dieback disease,” says Dr Monica Gerth from the University’s Centre for Biodiscovery and School of Biological Sciences. “If the spores can’t swim, they can’t make it to a kauri root to infect. These compounds could stop this pathogen from moving through soil and infecting kauri trees.”
These results came from a new collaboration between scientists and kaitiaki from iwi, Dr Gerth says, after colleague Chris Pairama (Te Taou, Ngati Whaatua, Waimauku) connected the research team with Ian Mitchell (Te Uri Taniwha, Ngāpuhi, Waima).
“Being from the north where kauri is common, Ngāpuhi have extensive knowledge about kauri and how plants interact with the forest, and we hoped that we could combine their mātauranga Māori and our scientific knowledge to address the serious problem of kauri dieback disease.”
She says Ngāpuhi knowledge and experience shows that a healthy forest involves three stages of plants— ‘first wave’ plants that cleanse and prepare the soil, ‘second wave’ plants that encourage fertility and growth, and ‘third wave’ plants, including kauri, that bring permanence and stability.
The research group studied four ‘first wave’ plants—kānuka, karamū, kawakawa, and nīkau—to see if the cleansing activity of these plants was due to anti-microbial properties, Dr Gerth says. In the end, testing showed that kānuka extract was most effective at stopping the pathogen.
Mātauranga Māori and scientific knowledge were combined at every stage of this project and collecting and testing the plants was a collaborative effort, Dr Gerth says.
“This project was about mutual trust and collaboration, and it was very important to us to create an ethical collaboration,” Dr Gerth says. “These plants are taonga to Māori, and therefore the right of mana whenua to practice kaitiakitanga (stewardship) should be acknowledged and respected.”
Dr Gerth and her colleagues hope to continue their search for new compounds, while also exploring how their findings can be applied to protect kauri trees in the field.
“Kauri dieback is one of the biggest crises ever to face New Zealand's forests. If we lose kauri, we lose not only a unique ecosystem, but also a key part of part of New Zealand’s identity, history and culture,” Dr Gerth says.
Kia mate te ngarara o te kauri, kia whakaora te mauri o te ngahere. Kauri ora, mauri ora!
This research was funded by the Ministry of Business, Innovation, and Employment, and published in the Journal of the Royal Society of New Zealand. The manuscript is freely available online at: http://dx.doi.org/10.1080/03036758.2019.1648303.
In addition to Dr Gerth, Mr Mitchell and Mr Pairama, the cross-disciplinary research team included Dr Scott Lawrence from the University of Otago, Professor Nigel Perry and Ms Elaine Burgess from Plant & Food Research, Associate Professor Wayne Patrick from Victoria University of Wellington, and Dr Amanda Black from Lincoln University.
Germs and geothermals—a uniquely New Zealand collaboration
Dr Rob Keyzers from the School of Chemical and Physical Sciences is leading a long-running, uniquely New Zealand research project to help find new sources of antibiotics.
10 June 2019
The collaboration is looking at a group of organisms called ‘extremophiles’—organisms that live in extremely hot or extremely cold environments unsuited to human habitation. For the past several years, the research—involving scientists from Victoria University of Wellington, GNS Science, the University of Auckland and the University of Canterbury—has focused on one organism that lives around geothermal vents in New Zealand.
And although the project has encountered many hurdles and setbacks, Dr Keyzers says they can successfully point to research spanning organism discovery through to synthesis.
“This project was all about the right people coming together in the right environment with the right resources, all of which were found in New Zealand,” Dr Keyzers says.
“It started in 2011 when I was looking for new sources of antibiotics in the natural world,” Dr Keyzers says. “Nature has been a wonderful source of antibiotics so far, but we always need new drugs that kill pathogens in new ways. Extremophiles were an ecological niche that hadn’t been explored much, so I thought it might be a good place to start looking.”
Dr Keyzers contacted Matthew Stott, formerly at GNS Science and now the University of Canterbury, who is an expert in growing bacterial extremophiles from geothermal environments.
They started looking at one particular extremophile for natural products that might lead to a new antibiotic. Dr Stott had recently sequenced the genome of that organism, which meant they could examine the DNA for sequences already known to be helpful in creating antibiotics.
“Matt’s organism had the potential to make four useful molecules written into its genetic code,” Dr Keyzers says. “We had my Master’s student, Emma Aitken, test the organism to see if any of these molecules were actually being produced, so we could test it for potential applications. She found one—a peptide that is part of a known class of antibiotics, which was very exciting.”
The next step was gathering enough of this molecule to test it for potential applications. This turned out to be more of a challenge than Dr Keyzers and his team were expecting—the organism would only grow under very specific conditions, and only produced a very small amount of the compound.
“Emma went through 1200 petri dishes to grow this organism,” Dr Keyzers says. “It would only make the molecule we wanted if we grew it in a petri dish on a certain type of agar. Even after that, we could only gather around 400 micrograms (0.4 g) of the molecule.”
Dr Keyzers began looking at options to synthesize the molecule and contacted Distinguished Professor Dame Margaret Brimble from the University of Auckland, a world-leading expert in synthetic chemistry. Coincidentally, Dame Margaret had also recently developed an interest in extremophiles.
“Dame Margaret and her team were able to synthesize the molecule using some very interesting chemistry techniques they had developed,” Dr Keyzers says. “They were only able to make a small amount, but it was enough to start testing the molecule.”
Unfortunately, the tests were not promising—the molecule didn’t seem to have any effect on the bacteria they tested it on. However, Dr Keyzers and his colleagues were only able to test the molecule against a small group of bacteria.
“The molecule could be a successful treatment for other bacteria we couldn’t test against, or it could be an anti-fungal,” Dr Keyzers says. “We would need to do further tests to find this out.”
Although the tests themselves were unsuccessful, Dr Keyzers says this research project has been hugely beneficial in other ways.
“We were able to get our work published in a well-regarded journal, Chemical Science, and put New Zealand on the map as a leader in this area of chemistry,” Dr Keyzers says. “There have been very few other cases where a research collaboration has been able to run the whole gambit from discovery to synthesis.
The research group also followed a vigorous identification method, which Dr Keyzers hopes will set a high standard in this field, and Dame Margaret and her team at the University of Auckland were able to develop several improvements to the synthesis process as well, Dr Keyzers says. They were also able to exploit an ecological niche—geothermal extremophiles—that is very New Zealand-centric and is an area where New Zealand can offer unique research possibilities in both geothermal and extremophile niches.
“It needed someone who knew about extremophiles and someone with knowledge of my area of chemistry, both of which are reasonably rare, as well as a microbiologist to provide material for me to test that I could then pass on to an expert in synthesis to create. We looked at a fairly unusual area with extremophiles and were able to achieve all these great things here in New Zealand.”
“Collaborations like this are one of the benefits of living in New Zealand,” Dr Keyzers says. “We have a small community of researchers here, brought together through the Maurice Wilkins Centre of Research Excellence, who all know each other and can easily work together, using New Zealand’s natural resources to push forward projects like ours.”
Dr Keyzers plans to continue his work on this project and hopes to bring in the expertise of School of Biological Sciences colleague, Dr Jeremy Owen. Dr Owen is a specialist in taking genetic codes that produce certain molecules from one organism and transplanting them into another organism to help them grow faster.
“Jeremy’s expertise can help us produce molecules faster, as well as take DNA from anywhere and grow it,” Dr Keyzers says. “Along with Margaret’s expertise in synthesis, we can now discover and grow potentially helpful molecules much faster, which is a very exciting prospect.”
The researchers acknowledge all governance entities representing owners and shareholders (tangata whenua) of Māori Freehold Lands of Aotearoa New Zealand, in this case Tikitere Trust, who have kindly consented to research and discovery being conducted on their land.
Antifungal activity of Feijoa brings Research Article of the Year Award to Centre for Biodiscovery PI
Many of us have heard of antibiotic-resistant bacteria, either through the media or perhaps knowing someone who died from such a bacterial infection. Just as there are bacterial infections resistant to antibiotics, there are fungal infections resistant to antifungal drugs.

21 May 2019
Fungi are microbial organisms (not visible to the naked eye) that can infect plants, animals and humans. Yes, mushrooms are fungi, but not all fungi are mushrooms. Approximately 300 fungal species are known to be pathogenic to humans; these include well-known species such as Candida albicans (the causal agent of vaginal yeast infection and oral thrush) as well as the numerous species that cause athlete’s foot.
Unfortunately, not all fungal infections are able to be treated successfully. Fungal infections cause approximately 1 million deaths per year, an alarming number that exceeds the annual deaths caused by breast cancer as well as those caused by malaria. And though people are not currently dying from vaginal yeast infections, oral thrush or athlete’s foot, there is potential for these fungal species to evolve resistance to antifungal drugs.
A recent article published in The New York Times (6 April 2019) profiled Candida auris, a fungus (specifically a yeast) resistant to antifungal drugs, spreading around the world killing a significant number of people since 2009. Prolonged exposure to high doses of antifungals (as in people with compromised immune systems such as the ageing senior population, cancer patients receiving radiation or chemotherapy treatment, and organ transplant patients), is a means for C. auris, and other fungi, to evolve resistance.
The current clinically-approved antifungal drugs are divided into four classes based on their mechanisms, or ways they work. This means a fungus has to overcome merely four mechanisms in order to become resistant to currently-available drug treatment. One potential solution to treating C. auris is combination therapy where lower doses of more than one drug will be used to treat an infection. However, this solution is limited as some drugs cannot be combined.
Developing new antifungals
For these reasons, it would be ideal to identify and develop a new antifungal drug that works by a new mechanism distinct from the current four classes of today’s drugs; this would add another hurdle in the path to resistance. Developing a drug that will target the fungus only and not affect the biology of uninfected cells in the body is not easy and is the reason why there is a shortage of antifungal drugs.
Nature is a proven source to discover the next generation of antifungal drugs as most existing antifungal drugs have been based on natural compounds. As C. auris has not yet been reported in New Zealand, our plants and soil may contain the key to an antifungal drug effective at combatting C. auris.
Research in my lab at Victoria University of Wellington, in collaboration with Drs Rob Keyzers and Michael Jackson as well as the feijoa breeder Nigel Ritson at Foretaste Feijoa Fruit Ltd (Takaka), has identified compounds in the peels of feijoa that inhibit the growth of Candida species that are closely related to C. auris; this work was recently published in The Journal of Agricultural and Food Chemistry. These compounds work by targeting fungal-specific molecules that are not targeted by the four classes of today’s antifungal drugs – thus it will be good to test these compounds against C. auris and other antifungal-resistant species.
Written by Dr Andrew Munkacsi
His recently published article has also won the Journal of Agricultural and Food Chemistry 2019 Article of the Year Award.
University grant recipients include vaccine to treat drug addiction
Victoria University of Wellington scientists developing ground-breaking new vaccines to treat drug addiction have received one of fifteen $150,000 Explorer Grants from the Health Research Council of New Zealand.

1 April 2019
Victoria University of Wellington-led research received three of the Explorer Grants for 2019, which were announced this morning.
Addiction to drugs of abuse such as nicotine, methamphetamine, cocaine and heroin could all be treated more efficiently and successfully as a result of the project led by Dr Benjamin Compton (pictured) from the University’s Ferrier Research Institute and including Dr Lisa Connor from the School of Biological Sciences.
The treatment they are developing incorporates an approach known as immunopharmacotherapy.
Addictive drugs are small molecules that easily cross people’s blood brain barrier and bind themselves to receptors, triggering reward signals. Using immunopharmacotherapy, a vaccine induces drug-specific antibodies that bind themselves to the drug, preventing it from crossing the blood brain barrier and acting on the central nervous system, thereby reducing its addictive effects.
“Despite advancements and many promising pre-clinical findings, decades of research investigating immunopharmacotherapy as a treatment option for drug addiction has not yet resulted in a vaccine candidate demonstrating efficacy in final clinical trials,” says Dr Compton.
“This funding from the Health Research Council will enable us to develop and assess an exciting new synthetic vaccine platform that could pave the way for the first efficacious immunopharmacotherapy for humans, profoundly changing the way physicians can medicate for drug addiction.”
It is envisaged the vaccine platform could be easily adapted to help treat multiple drug addiction disorders.
“Our research has the capacity to provide better outcomes for patients—including avoiding the side-effects associated with current anti-addiction medications—as well as reducing the burden harmful drugs have on society, which is estimated to cost the New Zealand economy $1.8 billion a year,” says Dr Compton.
In a second Health Research Council-funded project, Dr Wanting Jiao, also from the Ferrier Research Institute, is using the computational power of quantum and molecular mechanics to investigate a previously hard-to-access tuberculosis (TB) enzyme and design an antibiotic to fight it.
“We will make possible the development of a new generation of anti-TB drugs in a considerably shorter period of time and at greatly reduced cost than current methods allow,” says Dr Jiao, who is collaborating with scientists from the University of Otago.
The technique could also be used in the battle against other pathogenic bacteria, an international health priority due to the global rise of multidrug-resistant bacteria.
“Our novel computational methods will vastly improve the ability to design new classes of highly potent and selective enzyme-inhibiting antibiotics,” says Dr Jiao. “They will overcome the problems that plague existing techniques and promise to revolutionise drug design.”
The third Victoria University of Wellington project is led by Professor David Ackerley from the School of Biological Sciences and includes collaborators from the Malaghan Institute of Medical Research, based at the University, Johns Hopkins University in the United States, and the University of Auckland. The team aims to advance cellular regeneration research and degenerative disease modelling.
Dr Compton, Dr Jiao and Professor Ackerley are all active members of Victoria University of Wellington’s Centre for Biodiscovery.
The University’s Vice-Provost (Research), Professor Margaret Hyland, says they highlight the University’s commitment to improving health and wellbeing.
“The Ferrier Research Institute has a long and proud history of drug discovery and that continues with the two projects funded today. Other important health research is being conducted elsewhere in the University too, not least in other parts of the Faculty of Science, home to another of today’s supported projects, and in the Faculty of Health we established in 2017,” says Professor Hyland.
She adds that the three projects illustrate how Victoria University of Wellington researchers “collaborate across disciplines, institutions and indeed countries to incorporate wide perspectives in their endeavours and ensure the highest-quality results”.
Feijoas promise new anti-fungal treatments says Victoria research
8 December 2017
As well as tasting great, the humble feijoa may also offer new treatments for life-threatening fungal infections according to a Victoria University of Wellington researcher.
Mona Mokhtari will graduate with a PhD in Biomedical Science at a Victoria graduation ceremony next week, after conducting research into the antifungal properties of one of New Zealand’s favourite fruits.
Researchers have been interested in the feijoa’s antibacterial and anti-cancer potential for some time but Mona’s research is one of only a handful of studies into its antifungal properties.
“Fungal infections cause one million deaths per year worldwide—more than breast cancer or tuberculosis—and that’s even with the availability of antifungal drugs,” Mona says.
“The problem is many of the antifungal drugs doctors have relied on for years are becoming less and less effective as these infections build up antifungal resistance. That’s why we need to expand the range of antifungal drugs doctors have at their disposal.
“I became interested in feijoas, partly because New Zealand is so passionate about them, but also because they’re a source of natural products. Research has shown that drugs based on naturally occurring compounds often produce fewer side effects in patients and can be taken in lower doses than synthetic drugs.
“I worked with Foretaste Feijoa Fruit in the South Island to identify and test a particular compound in feijoas. I found that it is about 50 times more effective as an antifungal than as an antibacterial. That makes the compound very promising as the basis for a drug that kills fungal cells without hurting human cells or the beneficial bacteria in the guts of humans.”
Mona says a lot more work needs to be done before a drug can be developed and made available to doctors. For the time being, she is starting a new project looking at the anti-cancer and anti-diabetic properties of feijoas.
“Now that the compound has been identified and once the research has been published, other researchers have a head start on turning this compound into something you might see in pharmacies in years ahead,” Mona says.
Funding for research into Pasifika and Māori traditional remedies
22 November 2017
Dr Woolner, who will graduate with a PhD in Chemistry in December, received the funding through the Health Research Council’s Pacific Health Research Postdoctoral Fellowship programme. She will undertake the research over the next three years at the University’s Chemical Genetics Laboratory and with Dr Rob Keyzers and Dr Andrew Munkacsi as her supervisors.
Helen hopes her research will “elucidate the science behind the tradition” and help Māori and Pasifika people harness the full potential of their long-held natural health practices.
Helen, who is of Cook Island Māori descent, remembers her mother and grandmother using traditional plant-based medicines to treat minor ailments when she was a child. “But only when I started studying science 12 years ago, did I gain an understanding and an appreciation for my mum and grandma’s use of medicinal plants.”
As a starting point, she will draw on research by 2016 Victoria PhD graduate Dr Seeseei Molimau-Samasoni, who used biological and chemical tools to identify the iron-chelator compound in a Samoan plant that is traditionally used for its anti-inflammatory activity—like a natural ibuprofen.
“I will use biological and chemical experiments used by Dr Molimau-Samasoni, along with others, to track and further purify the active chemical component of a Samoan plant species known for its natural healing properties. Once in a pure form, I’ll be able to evaluate the compound for its potential activity against diseases associated with iron-overload that are of significant concern within the Pacific.”
Helen will then look for novel compounds that may produce healing properties in other selected plants from Samoa, the Cook Islands and New Zealand.
“These results will provide insight into the chemistry and biology of traditional medicine in the Pacific,” she says.
Dr Munkacsi says the chemical biology of Māori and Pasifika traditional medicine is poorly understood, especially when compared to traditional medicine in other parts of the world.
“Helen’s research could identify the compounds that have potential to be pharmaceutical drugs. Drugs that could do what the traditional medicine has been doing for hundreds or thousands of years, or something related or even something new.”
Dr Keyzers says Helen’s research feeds into the growing need for new medicines,
“Worldwide, there’s an understanding that biodiscovery of new medicinal compounds is vitally important. At the same time, we need to recognise that 80 percent of the world’s population rely on herbal (traditional) medicines. So understanding how these work, how efficacious the treatments are, and what unforeseen effects they may have, is of great importance.”
Helen will have additional research support from Hikurangi Enterprises, the Scientific Research Organisation of Samoa, Professor Anne La Flamme from Victoria University, and Professor Greg Cook from the University of Otago.
‘Silver bullet’ solutions in NZ antibiotic research
28 August 2017
Amidst the burgeoning threats of a global “antibiotic apocalypse” and a major New Zealand health crisis hospitalising more than 100 children every year, two leading researchers from Victoria University of Wellington are breaking new ground in the fight against pathogens and will introduce their work at an upcoming Spotlight Lecture focusing on antibiotics, drug discovery and penicillin prevention of rheumatic fever.
Developing novel antibiotics, including re-imagining the age-old use of silver to treat infection, is the focus of work by Dr Darren Day in Victoria’s Centre for Biodiscovery, while Dr Dianne Sika-Paotonu from the Faculty of Health is focused on a new formulation and delivery method of penicillin used for rheumatic fever.
Silver has been used for centuries to kill germs and bacteria, and even ward off or destroy evil, so its beneficial properties are widely accepted. But what Day and his team are working on is a ‘silver bullet’ in the form of an innovative method of using aptamers (synthetic antibodies) to deliver medicines to specific targets – in this case Pseudomonas aeruginosa, an “opportunistic bacterium” commonly infecting those with compromised immune systems that is ranked by the World Health Organisation in 2017 as the second greatest microbial threat to human health and in dire need of new antibiotics to treat it.
“Aptamers are essentially chemical antibodies which are selected to target specific pathogens. We have joined these aptamers targeted at P. aeruginosa to medicinal silver – which has been known since 4000 BC to be incredibly effective in treating infections,” says Day.
“In larger doses silver can be toxic, but what we have done with the aptamers is ensure specific delivery to the bacteria and not to the surrounding cells. These ‘aptabiotics’ are quick to produce compared to antibodies, drug molecules, including nanomaterials, can easily be incorporated into their structure to target specific cells, and they kill bacteria incredibly rapidly.”
Initial testing by Day and his team has proven highly successful, and the team is expanding trials with the cutting-edge process they have now patented.
“We’re the only ones doing this at the moment, and it’s a little bit out there. People never thought about delivering silver directly to the cells themselves. What we’re trying to do now is tailor these aptamers to other pathogens,” Day says.
Although the need for a new range of antibiotics to combat the ever-growing number of resistant bugs is essential, for diseases like rheumatic fever, penicillin is still the best response and preventative measure we have.
Considered an illness of developing countries, acute rheumatic fever (ARF) is an autoimmune condition caused by untreated group A streptococcal (GAS) bacterial infections of the throat (and possibly skin) which causes the heart, joints, brain and skin to become inflamed and swollen. Multiple or severe attacks of ARF can cause permanent heart damage known as rheumatic heart disease (RHD).
Painful monthly injections of the antibiotic Benzathine Penicillin G (BPG) are given for at least 10 years to prevent further GAS infections that can lead to ARF and cause RHD. New Zealand has high rates of ARF, with Māori and Pacific children and young people aged 5-14 years most affected.
Together with collaborators, Sika-Paotonu’s work is concerned with the ongoing prevention of ARF by reformulating the monthly penicillin injections required to prevent further GAS infections that could cause another bout of the condition.
“These monthly injections are needed for at least a decade and sometimes a lifetime, and by all accounts each injection is very painful. A new penicillin for ARF/RHD is urgently needed,” says Sika-Paotonu.
“A vaccine against GAS is on its way, but will take time, so we are looking at how to better manage this disease in the interim. Penicillin works great, but the injections are horrible. We are part of a global effort to reformulate BPG to make it less painful to give and hopefully last longer.”
Another important component of Sika-Paotonu’s research is finding out how BPG actually works in the bodies of those most affected.
“The initial studies were carried out in the 1950s to determine how BPG would work, but they gave injections to soldiers in the US who were all fit, healthy European men aged 18-24. The data was then used to determine how we use penicillin today on very different groups of people, including sick young people. Clearly there’s a huge gap in the research around this which we are also looking to fill,” Sika-Paotonu says.
“This is a major health issue in New Zealand, and globally, that we need to continue raising awareness about while we work to address it.”
- Newsroom
A tiny solution for fisheries’ big problems
27 July 2017

A tiny device to instantly detect pathogens that is being developed by researchers at Victoria University of Wellington could save the seafood industry millions of dollars a year and help reduce overfishing.
Professor Thomas Nann and Dr Renee Goreham, from Victoria’s School of Chemical and Physical Sciences, are working on a device that will use state-of-the-art aptamer technology to detect hazardous levels of food pathogens, specifically E. coli.
Professor Nann, who is also Director of the MacDiarmid Institute for Advanced Materials and Nanotechnology, says the device will target the extracellular vesicles (EVs) excreted by E.coli. These vesicles are nanometre-sized structures consisting of fluid enclosed by two layers of lipid molecules, which are released by cells.
“Once we’ve isolated the vesicles that are given off by E.coli bacteria—which is relatively easy to do with the nanotechnology we work with—we will then develop an aptamer [a molecule that binds to a specific compound] that will target them,” he says.
“It’s difficult to chase one single bacteria, because we cannot see it—at the moment we have to grow a sample in a lab over several days before we can actually identify if it contains bacteria. But bacteria excrete these vesicles all the time in great numbers, so if we chase the vesicles rather than the bacteria itself we can gather enough to detect it much more easily.”
He says the technique can be compared to finding a needle in a haystack. “The easiest way to find the needle is to take a magnet and run it over the haystack—we would be doing a similar thing, but instead of magnets we’d be using aptamers, which are a bit like chemical magnets. The vesicles we’ve identified would stick selectively to these aptamers.”
Dr Goreham says pathogen contamination is a huge problem in the food industry and can be costly—latest figures estimate the cost to New Zealand to be around $161.9 million a year.
“Fisheries are crying out for a fast, reliable and highly targeted sensor for foodborne pathogens. Currently, they have to monitor their whole manufacturing systems, which is very costly and not very reliable. Culturing a sample can take three days, so if that result comes three days after the initial contamination it could have spread throughout the entire factory in that time, meaning they have to shut down the whole operation,” she says. “We think that by detecting extracellular vesicles instead of the bacteria cells themselves, we will be able to identify pathogens on-site, which will make the process not only faster but much cheaper too.”
Dr Goreham says there will be other benefits too.
“Fisheries will save money by not having to dispose of a contaminated catch, recall the product or clean the entire processing plant, which they would otherwise have had to do after waiting several days for the presence of bacteria to be confirmed from a cultured sample,” says Dr Goreham. “As a consequence of not having to dispose of so much of the catch, more seafood will make it to market—that would mean larger profits for the fisheries as well as a likely reduction in overfishing.”
She says the technology has the potential to be applied to other food industries. “We are starting with fisheries, but once the prototype’s been developed and commercialised then the idea could be applied to industries such as milk or meat.”
Professor Nann and Dr Goreham are working with seafood company Sanford Ltd to test the technology. The pair is also hoping to work with AuramerBio, a specialist aptamer-producing company based in Wellington that was co-founded by Victoria University Associate Professor Justin Hodgkiss.
Professor Nann says the device could have a huge impact on the New Zealand economy. “We think it could enable the creation of high-value manufacturing jobs here, and also help lessen routine testing costs for the food industry, allow early in-house testing and reduce the large-scale wastage that comes with product recalls.”
‘We’ve reached peak antibiotics’
17 July 2017
Superbugs are one of the greatest threats to human health, and Kiwi researchers are using several pioneering methods to find new ways to help. Naomi Arnold reports.
When antibiotics entered widespread use 200 years ago, they changed our lives, giving us a weapon against common bacterial infections that can kill.
Since then, some bacteria have developed resistance to certain antibiotics, leading to the rise of superbugs, which are becoming a major problem in New Zealand hospitals.
A 2016 report by the Institute of Environmental Science and Research (ESR) for the Ministry of Health found New Zealand was one of the highest users of antibiotics in the developed world.
Many antibiotics can no longer be used, and on the horizon is the possibility that infections once thought conquered may kill again.
Contributing to the problem is the historical misuse of antibiotics: prescriptions for viruses, people failing to complete their courses of medication (thereby allowing bacteria to evolve to resist the antibiotics), their use in food and meat production, and antibacterial soaps in home and industrial cleaning products, which go straight into our waterways.
The World Health Organisation recently described humanity as being in “a race against time” to develop antibiotics against multi-drug resistant superbugs. According to one estimate, annual deaths from superbugs will reach 10 million by 2050.
But despite the threat of widespread resistance, research into new antibiotics has been declining since a golden age of discovery in the 1940s-1960s. However, several New Zealand scientists are searching for answers.
We simply need more money for research, says Dr Siouxsie Wiles, who Newsroom featured in May when she and CureKids launched a crowd-funding effort to pay for her testing of 1000 soil and fungi samples for their potential to kill superbugs. So far, the effort has raised 111 per cent of her $250,000 goal: nearly $280,000.
That’s a sentiment repeated by Massey University microbiologist and senior lecturer Dr Heather Hendrickson, who has been working in the field for 17 years.
“I would love to see more interest from funding agencies in this sort of work,” she says.
An age where humans die of common infections isn’t far off. “We’ve reached peak antibiotics.”
Hendrickson investigates a range of issues, including how bacteria evolve and the discovery of viruses which infect them, called bacteriophages.
“These are the ancient enemies of bacteria and they have huge potential as a tool to kill bacteria,” she says. “But we have barely scratched the surface of their diversity; they are still largely unknown.”
One of the processes she studies is horizontal gene transfer, where even distantly-related bacteria exchange DNA, including genes that are resistant to antibiotics. It can also result in new pathogens.
She says the work is of “critical importance”. New Zealand is seeing increased instances of antibiotic resistance in many of the same pathogens noted by the World Health Organisation.
She was recently part of the Royal Society of New Zealand review on the topic, which collected data that suggested New Zealand is witnessing increases in methicillin-resistant Staphylococcus aureus (a common cause of skin infections, sinusitis, and food poisoning); further resistances in some gut bacteria, like E. coli; and also those that cause sexually transmitted infections, like Neisseria gonnoroheae, responsible for gonorrhea.
With one of the issues in antibiotic resistance the use of the bacteria-killers in animal feed, Hendrickson says we need to start demanding meat be labelled with whether or not antibiotics are used during production.
“This is a step that is being considered in legislation elsewhere and I think it would make consumers more aware of the actual costs of the cheap meat that they are eating.”
Consumers can help by not buying antibacterial soaps, by not using antibiotics unnecessarily (e.g. for a virus). “We can all play a part in ensuring that antibiotics last for as long as possible.”
As for a solution, she says she’s interested in scientific projects that involve looking for new antibiotics. “I also think we need to consider approaches like phage therapy and phage therapeutics, where we use the natural enemies of phages and their products to fight bacterial infections where possible.”
At Victoria University of Wellington’s School of Biological Sciences, senior lecturer Jeremy Owen and associate professor and biotechnology programme director David Ackerley are searching for new antibiotics from bacteria that live in soil and other complex environments.
They are at the forefront of this particular type of synthetic biology approach to discovering new drugs.
“If we cannot find effective new antibiotics soon, we may be faced with a return to the 1920s pre-antibiotic era, where people routinely died of the most mundane things, like a scratch from a rose thorn while gardening,” David Ackerley says.
“The good news is, we have learned a lot over the past 70 years about how to better use antibiotics to slow the development of resistant bacteria. The bad news is that in that time we have burned our way through nearly all of the antibiotics discovered to date. We are trying to refill our pharmacies with new antibiotic options.”
The majority of antibiotics in use today were discovered by growing bacteria isolated from different soils around the globe, testing the different molecules they naturally secrete.
The pair say this was a highly productive approach between the 1940s and 1960s, but researchers have struggled to find anything new since.
“The same sets of molecules just kept cropping up time and time again,” Ackerley says. “In recent times we have realised that only a very small proportion of soil bacteria – under 1 percent – can be grown effectively outside of their natural environment. It is a certainty that the remaining 99 percent produce some very effective antibiotics that we have previously been unable to access.”
Because most soil bacteria can’t be grown in a lab, the team are going straight to the bacteria’s DNA, purified from the soil. They’ve developed several different strategies to ‘fish out’ clusters of gene that encode antibiotic-synthesising cellular machinery.
“These genes effectively act as blueprints that tell a cell how to make one particular antibiotic,” he says. “Taking advantage of the fact that bacteria are so good at swapping bits of DNA, we and others have shown that you can employ ‘synthetic biology’ approaches to transfer these blueprints to a new host – a bacterium that we can grow in the lab – and a surprising amount of the time it will gain the ability to produce a new antibiotic.
“Luckily for us, most antibiotic gene clusters not only encode the assembly line needed to make an antibiotic, but also a means for defending the host cell against any toxic effects, so the new bacterial host is usually immune to the new drug it is making.”
He says big pharma companies seem to be increasingly interested in this space, which bodes well for downstream development of promising new drug candidates.
Four questions for Victoria University of Wellington's David Ackerley
Are we doing enough to tackle this global problem?
No. Not yet. Until very recently there has been little incentive for large pharmaceutical companies to develop new alternatives to current antibiotics, as any new antibiotics to hit the clinic will likely be reserved to treat only the cases where the current frontline drugs fail.
Because antibiotics work so well in curing disease then patients don’t need to keep taking them for years on end (in fact, good clinical practice will do what it can to discourage unnecessary use). All of this reduces profit margins for the pharmaceutical companies.
However, there are starting to be “prize-type” financial incentives implemented by governments to encourage discovery, moreover the fact is the situation is starting to get really dire, so the market for new antibiotics that are effective against drug-resistant superbugs is unfortunately growing all the time.
How is your work funded?
Our main source of funding at present is a $1.2m grant from the Health Research Council of NZ, specifically for discovery of new antibiotics. But one of our key methods for finding promising gene clusters came out of an unrelated ‘blue skies’ Marsden project, which really emphasises the importance of funding basic research to enable unexpected discoveries.
We have also received supporting funding from the Maurice Wilkins Centre for Molecular Biodiscovery and the Cancer Society of NZ, to try and find promising anti-cancer drugs in a similar manner.
Coming back to the basic discovery level, it’s really hard to get funding for a project that wants to search for new drugs – the fight for scientific research funding is so competitive that grant review panels tend to strongly favour projects with a logical and clear progression of goals and a high likelihood of success.
They usually don’t like projects where the first aim is to find new compounds, as all the remaining aims will be 100 percent dependent on the first one, and if that is not successful the whole project falls over. The phrase commonly used to dismiss such projects is “that’s just a fishing expedition”, and to get past that criticism you usually have to convince the panel your methods are incredibly new and exciting, and have a high likelihood of success.
Otherwise, you are faced with the chicken-and-egg scenario that you can’t get the funding without having already found the new drug candidates, after which you no longer need the funding for the discovery work.
Given the severity of the problem, it does seem that having a few decent-sized grants reserved to specifically target discovery of new antibiotics might be warranted.
Who needs to make changes to ensure we are looking at ways to solve this issue?
At an academic or small company discovery level, government policy decisions can have a big impact on priority areas for funding. For drug development, governments can again play a key role in incentivising companies to develop new antibiotic drugs even if they will be reserved only to treat the most severe drug-resistant cases.
Large philanthropic organisations like the Gates Foundation can also potentially help provide incentives in the form of prizes etc for new clinically-approved antibiotics that won’t have a large patient pool, so that companies have a way of recouping the many hundreds of millions of dollars of investment it takes to bring a new drug the whole way from discovery to large and very expensive clinical trials, and ultimately to market.
How far off is a “solution” to the problem of antibiotic resistance, and what might that look like?
The preclinical and clinical trials needed to ensure new drugs are both safe and effective are not only super-expensive, they also consume large amounts of time. The problem is getting so severe that it is possible the next generation of antibiotics will be rushed through expedited trials in only a few years rather than the more usual decade or so – however, that kind of approach of course brings risks in that any side-effects or possible longer-term consequences of any approved drug will be less fully understood.
At any rate, the good news is that there are new types of antibiotics starting to come through the pipelines again. But we are nevertheless going to be faced, at least for a while, with increasing numbers of infectious diseases that are not safely treatable if they are even treatable at all.
Victoria scientist wins Supreme Award
17 July 2017

Professor Furneaux, Director of Victoria’s Ferrier Research Institute, was presented with the award for overall excellence in all core areas of research commercialisation at a ceremony in Auckland.
He also took home the Baldwins Researcher Entrepreneur Award, which recognises a researcher who has made outstanding contributions to business innovation or has created innovative businesses in New Zealand through technology licencing, start-up creation or by providing expertise to support business innovation.
Professor Furneaux has been recognised for entrepreneurial endeavours that have generated tens of millions of dollars of economic activity for New Zealand over the past 25 years.
Starting out as a synthetic chemist, today Professor Furneaux leads a team of 40 scientists at the Ferrier Institute, whose innovative medical drug compounds have been licensed to international pharmaceutical and agrochemical companies, and an exciting new start-up.
The judges described Professor Furneaux as “a world class research entrepreneur”, and his story as “one of enormous achievement”.
“It’s a real honour to receive these awards for myself and our talented team of scientists and collaborators,” says Professor Furneaux. “Also, a big shout out to the commercial partners who successfully applied our science.”
The Institute’s most successful commercial deal, in conjunction with Albert Einstein College of Medicine in New York, is its 16-year relationship with United States-based, NASDAQ-listed company BioCryst Pharmaceuticals, Inc.
Under this licensing deal, four generations of novel compounds, covered by over 160 granted patents, have yielded six lead drug candidates with applications as diverse as cancer, gout, psoriasis, transplant rejection and malaria.
One of these candidates is an active ingredient behind a new oral drug, Mundesine®, which treats patients with a specific type of non-Hodgkin lymphoma. In March this year, Japan became the first country to approve Mundesine®, licensed by BioCryst Pharmaceuticals Inc. under an exclusive licence with Albert Einstein College of Medicine and Viclink, Victoria University’s commercialisation office.
Professor Furneaux says he’s thrilled that his team’s successes with BioCryst spurred significant commercial benefit to New Zealand through the establishment of GlycoSyn, a Wellington-based manufacturer of pharmaceutical ingredients.
“We are always looking for areas where we can apply our chemistry in ways that differentiate us so that we can patent the intellectual property we create for the future benefit of both Victoria University and New Zealand as a whole.”
Collaborations key in Victoria’s commercial success
22 May 2017

Victoria University of Wellington is celebrating its success in science and innovation with two finalists in the 2017 KiwiNet Research Commercialisation Awards.
Professor Richard Furneaux, director of Victoria’s Ferrier Research Institute, has been named a Researcher Entrepreneur finalist, and Viclink, Victoria’s commercialisation office, is a finalist in the Commercial Deal category.
Professor Furneaux has been recognised for his entrepreneurial endeavours which have generated tens of millions of dollars of economic activity for New Zealand over the past 25 years.
Starting out as a synthetic chemist, today Professor Furneaux leads a team of 40 scientists at the Ferrier Institute, whose innovations include the synthesis of an active ingredient in anti-lymphoma drug Mundesine®. Last month, Japan became the first country to approve Mundesine®, licensed by BioCryst Pharmaceuticals Inc. under an exclusive licence with Albert Einstein College of Medicine and Viclink.
Research by the Ferrier team has also led to a breakthrough synthetic vaccine to treat cancer, allergies and autoimmune diseases. The Institute recently announced a five-year, $500,000 research partnership with the Breast Cancer Foundation New Zealand, which will see Ferrier scientists progress a potential breast cancer vaccine.
The Baldwins Researcher Entrepreneur Award recognises an entrepreneurial researcher who has made outstanding contributions to business innovation or has created innovative businesses in New Zealand through technology licencing, start-up creation or by providing expertise to support business innovation.
Viclink has also been named as a finalist for KiwiNet’s PwC Commercial Deal Award.
Viclink and the University’s Ferrier Institute have maintained a successful, 16-year relationship with United-States based NASDAQ-listed company BioCryst.
In conjunction with partners at Albert Einstein College of Medicine in New York, the licensing deal with BioCryst has resulted in more than 160 patents and six lead drug candidates with applications as diverse as cancer, gout, psoriasis, transplant rejection and malaria.
The relationship with BioCryst has yielded significant commercial benefit to New Zealand, the flow on creation of research jobs, and the establishment of GlycoSyn, a Wellington-based manufacturer of pharmaceutical ingredients.
Viclink has played a key role in the relationship between Victoria University and BioCryst.
The PwC Commercial Deal Award celebrates excellence in research commercialisation delivering outstanding innovation performance and the potential for generating significant economic impact for New Zealand.
KiwiNet is a consortium of fifteen universities, crown research institutes and a crown entity established to boost commercial outcomes from publicly-funded research.
The winners will be announced on Thursday 13 July in Auckland.
® MUNDESINE is a registered trade mark (in Japan) of Mundipharma AG.
Is mitochondrial transfer a player in bone marrow transplantation?
04 May 2017
Our Cancer Cell Biology researchers are undertaking a study that Group Leader, Professor Mike Berridge describes as “a world-first”. They are investigating whether DNA can transfer between cells damaged in bone marrow transplants.
“Using our cancer model, we showed that a damaged cell could collect fresh mitochondria from the host organism – there was a transfer of DNA,” said Dr Melanie McConnell, Malaghan Institute Research Associate and Senior Lecturer at Victoria University. The team soon realised that a similar situation occurred in bone marrow transplants. There, a patient is given therapies to suppress the growth of abnormally proliferating cells, before receiving replacement bone marrow from a donor. The result is that a transplant recipient could be left with two different types of mitochondrial DNA – their own, and that of the donor.
Using the differences between these mitochondrial DNA–there are about 40 base differences between any two humans – Prof Berridge and his research group aim to investigate whether genes travel between cells in order to replace those damaged in bone marrow transplants that include cancer. Eight donor-recipient pairs will be involved in this ground-breaking study: Samples of each participant’s bone marrow will be taken before, and again three months after transplantation. The aim of this is to examine whether any donor mitochondrial DNA markers are present in the recipient’s bone marrow.
In parallel, the team will investigate mitochondrial transfer in mice that have been treated with radiation – similar to that used in cancer treatment – which induces damage in the bone marrow. “We work with mouse models as it allows us to carefully design our experiments,” explains Prof Berridge, “…and to probe for genetic differences, we are using DNA sequencing and bioinformatics.”
Combined, these studies will provide a unique insight into the mechanism behind DNA transfer, and may have an impact on future treatment choices.
The Great New Zealand Trek: 9 Years and more than NZ$250,000 of support for our Multiple Sclerosis research
4 May 2017
With an impressive 238 participants taking part this year, it’s been another successful stage of the Great New Zealand Trek (GNZT): This year’s Stage 12 held earlier in March 2017, from Burkes Pass area just west of Fairlie to Becks in the heart of Central Otago, raised another $36,000, bringing the total support from the charitable trust to over $250,000.
Professor Anne La Flamme, who leads our Multiple Sclerosis (MS) research programme also participated in the trek, having done so since 2010. Her 12-year-old daughter Josie joined her this year for the first time.
Thanks to the generosity of the GNZT we are able to pursue novel and cutting-edge ideas. Professor Anne La Flamme says: “Most times these ideas are risky and they might not work, but they could prove enormous beneficial if they do. Because of the risk, it is incredibly difficult to find the funds to start this research, but having funds from the GNZT gives us that ability.”
The key achievements in our MS research have been in identifying several new therapeutic strategies to treat progressive MS and seeing them undergo clinical trials.
Kitty Johnson, a trustee and the organiser of the GNZT supports our MS research. “Professor Anne La Flamme’s engagement and the research team’s ability to think outside the box and probe new never-before- seen scientific discoveries is what it makes exciting and fundamentally important at the same time.”, she summaries.
For more information on The Great New Zealand Trek and to find out how you can get involved in Stage 13 in March 2018, please visit the GNZT’s website.
Driving the next generation of cancer immunotherapy treatments in New Zealand
4 May 2017
Professor Ian Hermans, Vaccine Therapy Programme Leader, and Dr Robert Weinkove, Wade Thompson Clinical Research Fellow and Clinical Director of the Human Immunology Lab, are establishing a research group that will bring cutting-edge new cellular therapies into New Zealand. This research involves a breakthrough area of oncology called CAR-T cell immunotherapy.
In this transfusion-like therapy, some of the patient’s own immune cells, the ‘T cells’, are modified to express a specific receptor – a chimeric antigen receptor (CAR) – in order to redirect them against cancer cells. “The approach works differently to vaccines, which aim to boost someone’s own immune response,” explains Dr Weinkove. “Here, we’re directly altering the immune cells themselves to target them.”
Central to the success of this new translational research is the expertise and knowledge of our team in good manufacturing practice (GMP) – international regulations for the production of medicinal products. “Our collaborators have developed an exciting pipeline of CAR-T cell therapies, our role is to make changes to the way they are manufactured and trialled, so that it fits with what’s regarded in the Western regulatory environment as ‘best practise’,” Prof Hermans explained.
For us at the Malaghan Institute, the driving motivation behind this project is the impact that it could have on the lives of New Zealanders. “For some leukaemias, more than half of people treated with CAR-T cell therapies have remained in remission for years without any other treatment,” Dr Weinkove said. “This is preliminary data, and we still have questions about the longer term effects, but as a clinician, I am extremely excited about the potential of CAR-T cell therapies.”
Victoria research leads to new drug for hard-to-treat lymphomas
19 April 2017

Japan has become the first country to approve an anti-lymphoma drug developed following initial research from Victoria University of Wellington.
The new oral drug, called Mundesine®, treats patients with a type of lymphoma called peripheral T-cell lymphoma (PTCL) — a group of aggressive diseases that accounts for 10 to 15 percent of all cases of non-Hodgkin lymphomas.
The active ingredient in the drug Mundesine®, forodesine hydrochloride, was first synthesised by Professors Peter Tyler and Richard Furneaux at Victoria’s Ferrier Research Institute, and first conceived by long-time collaborator Professor Vern Schramm from the Albert Einstein College of Medicine in New York.
The drug has been approved by Japan’s Ministry of Health, Labour and Welfare following 19 clinical trials.
“I’m very proud,” says Professor Tyler. “We’ve been working on this science for 20 years, and used a rational approach to design this drug. We resolved some complex chemistry and it’s great that, following this approval, the drug is now a step closer to being available.
“In some cancers, like lymphoma, T-cells, a type of white blood cell, replicate uncontrollably. This drug inhibits the enzyme PNP (purine nucleoside phosphorylase), causing a metabolic imbalance in the T-cells that triggers cell death. The approval of Mundesine® provides further treatment options for patients with PTCL.”
Mundesine® was licensed by BioCryst Pharmaceuticals Inc., under an exclusive licence with Albert Einstein College of Medicine and Viclink, Victoria University’s commercialisation office. BioCryst subsequently entered into an exclusive sub-licensing agreement with Mundipharma to develop and commercialise forodesine in the field of oncology.
The drug has been specifically approved for patients whose PTCL has relapsed (recurred) or is refractory (resistant to treatment). Few effective treatments have been available for these conditions. Those PTCL patients who relapse following chemotherapy currently live an average of only six more months.
The research to identify the active ingredient (forodesine hydrochloride) of the Mundesine® drug product was carried out with funding support for the Ferrier Research Institute from New Zealand government agencies, and for the Albert Einstein College of Medicine from the United States General Medical Institute of the National Institutes of Health.
® MUNDESINE is a registered trade mark (in Japan) of Mundipharma AG.
Five-year research partnership targets breast cancer vaccine
21 March 2017
A vaccine for breast cancer is on the horizon, thanks to a new partnership between Victoria University of Wellington’s Ferrier Research Institute and the Breast Cancer Foundation New Zealand (BCFNZ).
The five-year research partnership will see BCFNZ give the Ferrier Institute $500,000 to progress a significant breakthrough made by chemists at the Institute to create a life-saving breast cancer vaccine.
Ferrier Research Institute chemists are making gains in the area of cancer immunotherapy—described by leading journal Science as the ‘Breakthrough of the Year’ in 2013.
The Institute is developing a synthetic cancer vaccine technology that can activate tumour-specific T cells, producing a targeted immune response. This synthetic cancer vaccine causes rejection of cancer in several types of animal models.
Ferrier Institute director Professor Richard Furneaux says the technology is almost there. “We just need to get it to the next level of testing—human clinical trials.”
Professor Gavin Painter, who leads the chemistry team at Ferrier, says the support of BCFNZ is crucial.
“Getting a new therapy to human clinical trials requires significant investment, and an intensive campaign of chemistry, biology and regulatory compliance.
“Our success to date has been made possible because we work with the exceptional immunology research group led by Professor Ian Hermans at the Malaghan Institute of Medical Research here in Wellington, a relationship built up in a seven-year strategic collaboration.”
Evangelia Henderson, chief executive at BCFNZ says: “We went looking for a research partner who would give us the best shot of moving toward our vision of zero deaths from breast cancer. We were blown away by the calibre of the Ferrier team, the work they’d already done in the exciting field of immunotherapy and vaccines, and the strength of their international partnerships. It was a no-brainer for us.”
Cancer immunotherapy has caused a paradigm shift in cancer treatment, with a focus on targeting the body’s own immune system to fight cancer cells rather than introducing toxic agents to attack tumours directly. This line of research has led to the production of cancer vaccines which are showing promising results when used in certain situations; they are well tolerated by the body, have fewer side effects than current chemotherapy treatments and may be more effective in the long-term.
The successful immunotherapy treatment platform pioneered at Ferrier in collaboration with the Malaghan Insitute of Medical Research, has led to the establishment of biotechnology company Avalia Immunotherapies, which aims to commercialise the vaccine technology to help patients. Avalia’s chief executive officer is Victoria alumna Dr Shivali Gulab, a former NZBIO Young Bioscientist of the Year who is based in New York driving the progress of the vaccine technology towards human clinical trials.
For 20 years the Ferrier Research Institute has had an extensive working relationship with the Albert Einstein College of Medicine in New York which has resulted in successful drug trials. These include some of the most powerful enzyme inhibitors ever reported including Forodesine as a targeted therapy for a variety of haematological cancers, and Ulodesine as an orally available drug to treat severe gout.
Mimicking evolution to treat cancer
8 March 2017

Artificial forms of evolution are being used by a Victoria University of Wellington scientist to improve the ability of microbes to attack tumours.
Research led by Associate Professor David Ackerley, director of Victoria’s Biotechnology programme, has underpinned the development of a new form of chemotherapy that exclusively targets cancer cells.
A key goal of this chemotherapy is a more targeted treatment method that results in fewer side effects for cancer patients.
To achieve this goal, Associate Professor Ackerley and his team engineered enzymes that can transform a relatively safe and non-toxic compound (a “pro-drug”) into a drug that is highly toxic to cancer cells.
The genes encoding these enzymes are delivered to cancer cells using viruses or bacteria that are only able to replicate in tumours.
The pro-drug the team worked with is called PR-104A, and was developed by scientists at the University of Auckland, including Associate Professor Ackerley’s collaborators on this study, Associate Professor Adam Patterson and Dr Jeff Smaill.
“The enzyme we started with was moderately active with PR-104A,” says Associate Professor Ackerley. “However, this was purely by chance—nature has never evolved enzymes to recognise these very artificial types of molecules.
“We reasoned that by mimicking evolution in the laboratory—by introducing random mutations into the gene encoding our target enzyme, then selecting the tiny minority of variants where chance mutations had improved activity—we might eventually achieve a more specialised enzyme that could more effectively activate PR-104A.”
Not only is the team’s artificially evolved enzyme significantly better at activating PR-104A within living cells, it also addresses another major problem—how to keep track of the microbes in patients to make sure they are only infecting cancerous cells.
“A unique aspect of our work is that our enzymes can also trap radioactive molecules called ‘positron emission tomography (PET) probes’,” says Associate Professor Ackerley. “We hope that this will allow a clinician to put a patient in a full body PET scanner to safely identify the regions where the microbes are replicating.”
The team’s research has been published in this month’s edition of high-profile research journal Cell Chemical Biology, and has been supported by several New Zealand funding agencies including the Marsden Fund managed by the Royal Society of New Zealand, the Health Research Council of New Zealand and the New Zealand Cancer Society.
In ongoing work, Dr Smaill and Associate Professor Patterson have been developing more effective pro-drugs to partner with Associate Professor Ackerley’s enzymes. The team has been collaborating with groups at the University of Nottingham in the United Kingdom and Maastricht University in the Netherlands, aiming to progress the therapy into clinical trials in cancer patients.
Harnessing hope to treat rare disease in children
24 January 2017
New research from Victoria University of Wellington has provided insight into the effectiveness of a potential drug to treat a rare, fatal disease in children.
ometimes referred to as childhood Alzheimer’s, Niemann-Pick Type C (NPC) disease is a genetic disorder characterised by an inability of the body to transport cholesterol and other fatty substances (lipids) inside cells in the brain and liver. Lipids consequently accumulate in the liver, spleen and brain.
The disease causes quick and progressive mental and physical deterioration with typical loss of life prior to adolescence. Though rare, an estimated 500 children currently suffer from NPC disease worldwide.
An international team, led by Dr Andrew Munkacsi from Victoria’s School of Biological Sciences and Dr Stephen Sturley at Columbia University Medical Center, investigated the therapeutic efficacy of Vorinostat—a drug approved to treat cancer—to treat NPC disease.
“As no cure exists, finding drug therapy is crucial,” explains Dr Munkacsi. “Given that it takes more time to develop a new drug than the average lifespan of an NPC patient, identifying an existing drug treatment is the goal.”
“Vorinostat is currently in a clinical trial for NPC disease. This clinical trial was approved without some therapeutic efficacy testing because the drug was already approved to treat cutaneous T-cell lymphoma and potential drugs to treat NPC disease are in very high demand.”
In 2011, Drs Munkacsi and Sturley published a paper that identified the potential of Vorinostat to treat NPC disease. The study involved experiments using yeast as a model of NPC disease and then translating these experiments to cultures of human cells.
A new paper, published online last month in The Journal of Biological Chemistry, builds upon the 2011 study.
It found Vorinostat has the ability to correct multiple pathophysiological defects in the liver of an animal model that closely mimics human patients. The accumulation of lipids in liver cells was reduced and liver health was improved.
“By measuring the expression of approximately 14,000 genes in the liver, we were able to determine that Vorinostat normalised expression of key genes in the biosynthesis and transport of cholesterol in the liver,” says Victoria University Master’s graduate Natalie Hammond, a co-author on the new paper.
“It’s an exciting result that shows the potential of Vorinostat to treat NPC disease in the clinical trial”, says Dr Munkacsi.
“While it helped reduce lipids in the liver, it is unable to cross the blood-brain barrier and so may not address the build-up of lipids in the brain.”
Other contributing authors to the paper were from Callaghan Innovation in Lower Hutt, Tottori University in Japan and various research institutions in the United States (Mount Sinai School of Medicine, Washington University School of Medicine, Giesel School of Medicine at Dartmouth and University of Texas Southwestern Medical Center).
The study was primarily supported by the National Niemann-Pick Disease Foundation, the Ara Parseghian Medical Research Foundation and Dana’s Angels Research Trust.
“These parent-patient funded foundations are critical to investigations in rare diseases like NPC disease,” says Dr Munkacsi.
Research for Life grants for Centre for Biodiscovery researchers
7 November 2016
Five Victoria University medical researchers have received grants in the second funding round of the year from Research for Life, formerly known as the Wellington Medical Research Foundation.
Research for Life funds innovative quality research undertaken by researchers in the early stages of their careers who, through their work, will advance the quality of healthcare in the Wellington region and beyond.
Research Grants received are awarded to undertake innovative medical research. The successful applicants from Victoria for Research Grants include:
- PhD student Carl Beyers received a $10,000 grant to assess the impact of MIS416 therapy in altering the immune system of people with progressive multiple sclerosis (MS). There is an urgent need for therapies which are effective at treating progressive MS. MIS416 has been shown to be safe to use in progressive MS, and a large stage two clinical trial offering MIS416 as treatment is progressing. This grant is in aid of work to define how MIS416 alters inflammation at a cellular level. Access to samples from the trial, a valuable resource, offers an excellent opportunity to further medical research into MS treatments.
- Dr Darren Day, a senior lecturer in Victoria’s School of Biological Sciences, received a $20,250 grant to undertake research into developing a new type of anti-microbial drug for treating bacterial infections that are resistant to current antibiotics. Dr Day’s research uses DNA molecules coated with tiny particles of silver to specifically bind to bacteria and sensitize them to conventional antibiotic therapy.
- PhD student Kathryn Hally received a $25,833 grant to investigate platelet activation in acute coronary syndromes (ACS), the leading global cause of death. Anti-platelet medication is the standard-of-care for ACS patients but a proportion of patients will continue to experience recurrent cardiovascular events despite treatment. Ms Hally’s research focuses on how platelets may be alternatively activated in responses to infection and how this may relate to patient outcome.
- Professor John Miller from Victoria’s School of Biological Sciences received an $18,000 grant to undertake research into the development of new anti-cancer chemotherapeutics. Current drugs for solid tumours have unwanted side effects, and cancer cells can acquire resistance to these drugs. New drugs that are better tolerated and can improve patient outcomes are needed to either replace or be used in combination with those now in use.
Research for Life Travel Grants assist local researchers meet the cost of presenting their research findings at international conferences. A Travel Grant was awarded to:
- Jennifer Soundy, a third year PhD student at Victoria University, received a $2,000 travel grant to present her research findings at the International Society of Aptamers conference in Oxford in April next year. Ms Soundy is undertaking a study on the development of novel antimicrobials for fighting antibiotic-resistant bacterial infections.
Research for Life president Professor John Nacey says, “Research for Life congratulates the successful applicants of this round of funding. The research they are undertaking is innovative, well-conceived and vital to achieving continuing improvements in health outcomes in the community.”
A man on a drug discovery mission
27 September 2016

Professor Peter Tyler from Victoria’s Ferrier Research Institute’s career in chemistry spans 35 years—and 32 patents for potential drug candidates.
“Drug discovery is absolutely feasible in New Zealand, and we are doing world class research,” says Professor Tyler. “I really relish working on something that I know will ultimately be useful, and provide much needed treatments.”
As he will explain in his upcoming inaugural lecture, when chemistry is partnered with biology, real progress can be made.
“I’ve worked on a number of research projects that have resulted in promising drug candidates. This includes Forodesine, which has been through several clinical trials for T-cell cancers, and Ulodesine, which has successfully completed a phase two clinical trial for gout.
“One of our compounds has also been shown to have efficacy against malaria. Others have demonstrated activity against the disease visceral leishmaniasis—which is a particular problem in Brazil. Another compound is in preclinical development against solid tumours, and has also shown broad activity against several types of cancer.
“We are looking to develop new compounds that target trypanosome parasites—parasites which are the cause of Chagas disease in central and South America, and African sleeping sickness.”
Professor Tyler will also talk about his research into a potential treatment for Alzheimer’s disease, which recently received more than $850,000 in funding for its development. “Our drug candidates for Alzheimer’s disease are significantly different from others—no one else in the world is using this approach.”
The chemistry created during this research is now being extended into a three-year research study targeting cellular communications, supported by the Marsden Fund.
A Victoria graduate for his Bachelor’s degree and PhD, Professor Tyler joined the University in 2014 when the Ferrier Research Institute was formed. Prior to that, he worked as a scientist at Callaghan Innovation, and the former Industrial Research Limited and Department of Scientific and Industrial Research.
Million-dollar funding to fight antibiotic resistant superbugs
15 June 2016

Victoria University of Wellington researchers have been awarded nearly $1.2 million in funding to find new and improved antibiotics from previously untapped sources.
The Health Research Council of New Zealand has granted $1,195,267 to Dr Jeremy Owen and Associate Professor David Ackerley from Victoria’s School of Biological Sciences for their three-year project.
“The project will use DNA sequencing and synthetic biology to discover new drugs. These techniques allow us to extract new molecules from bacteria that can’t be grown in the laboratory,” says Dr Owen.
“Just because we can’t grow a bacterial species in the lab doesn’t mean we can’t access an antibiotic it makes. The instructions for how to build that antibiotic will be somewhere in its DNA—if we can find these instructions, we can make the antibiotic.
“Currently, scientists can culture less than one percent of bacteria that exist on Earth and this one percent has provided most of the antibiotics we currently use in medicine. But resistance to these antibiotics is spreading, so we need to turn to the unculturable bacteria to find new drug candidates.”
Associate Professor Ackerley says antibiotic resistance is a significant threat, with the World Health Organization recently describing humanity as being in a race against time to develop antibiotics against multi-drug resistant superbugs.
“We’re in danger of going back to the time when people would routinely die of the most mundane things, like infected scratches from rose thorns while gardening.
“Our work aims to discover new molecules that have antibiotic activity and it is our hope that these will be developed into new medicines. We desperately need new antibiotics to fight drug resistant bacteria but we also need to use these antibiotics more responsibly to prevent the development of resistance.”
Once the new molecules are tested for antibacterial, anti-fungal or anti-cancer properties, the team plans to take promising molecules forward as new drug candidates.
“So many promising drug candidates never make it to the clinic because there is not enough supply,” says Associate Professor Ackerley.
“Our synthetic biology approach ensures we will be able to make lots of whatever we find. Plus, the classes of molecule we are looking for do generally have strong antibiotic potential, so we think we have a good chance of finding something useful.”
Dr Owen and Associate Professor Ackerley will work with Dr Rob Keyzers and Associate Professor Peter Northcote in Victoria’s School of Chemical and Physical Sciences.
Professor Mike Berridge from the Malaghan Institute of Medical Research (based at Victoria's Kelburn campus) was also awarded just over $1 million of funding. Professor Berridge will work with Dr Melanie McConnell from Victoria University and Professor Mark Hampton from the University of Otago, Christchurch to investigate the transfer of mitochondria between brain cells.
Multiple sclerosis trial begins at Wellington Hospital
13 June 2016

A Victoria University of Wellington researcher is leading a clinical trial that may offer new hope to multiple sclerosis (MS) sufferers.
The trial will test two commonly used antipsychotic medications in secondary progressive MS. This form of the disease, for which there is currently no effective treatment, affects over one-third of all MS sufferers and causes significant life-long disability.
The trial, based at Wellington Regional Hospital, is actively recruiting for participants.
Victoria University immunologist Professor Anne La Flamme says repurposing medications is common for treating MS.
“The majority of agents used to treat the most common form of MS—relapsing remitting MS—were originally used for something else, like viral infections and leukaemia.
“We’re looking at two medications, clozapine and risperidone, which were designed to treat a variety of health disorders such as schizophrenia, bipolar disorder and autism.
“Clozapine and risperidone have always been targeted to mental illness but our studies show they are able to tone down the immune system in the brain, which is what causes MS, and this anti-inflammatory action is promising.”
Professor La Flamme is working with neurologist Dr David Abernethy from Capital & Coast District Health Board, and Associate Professor Bronwen Connor from the University of Auckland.
“The trial will be randomised, blinded and placebo-controlled, to closely monitor any potential adverse effects from the drugs as well as measure any changes to MS disease,” says Professor La Flamme.
The study has been funded by the Ministry of Business, Innovation and Employment and supported by the Neurological Foundation of New Zealand and the Great New Zealand Trek Charitable Trust. Professor La Flamme has also received additional funding from a Research for Life grant to investigate how these medicines affect the immune system during secondary progressive MS.
For more information about enrolling in the trial contact Liz Goode, trial nurse, on liz.goode@ccdhb.org.nz.
PhD student’s revolutionary cell research to fight brain diseases
27 April 2016
A Victoria University of Wellington PhD student hopes his Neurological Foundation of New Zealand scholarship will lead to new treatments for brain diseases.
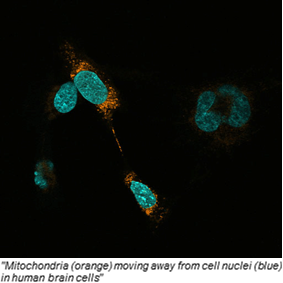
Matt Rowe received a 2015 Neurological Foundation W and B Miller Postgraduate Scholarship worth just over $100,000 to carry out research on mitochondria—the tiny structures that generate the energy to power a cell.
The research is likely to have implications for treatment strategies for degenerative brain disease like Alzheimer’s as well as brain cancer.
While it has been known for several years that mitochondria can transfer between cells, the reasons why a cell might give or receive mitochondria are largely unknown.
Matt’s research, carried out under the supervision of Dr Melanie McConnell from Victoria’s School of Biological Sciences and Professor Mike Berridge from the Malaghan Institute of Medical Research, will help to determine the drivers of this phenomenon.
“We’ll examine the mechanisms of mitochondria transfer in diseased cells in response to injury. We’re interested in the survival mechanisms of brain cells, what they do when injured, and how this relates to the movement of mitochondria,” says Matt.
A team including Dr McConnell and Professor Berridge have found that cancer cells will acquire mitochondria from the normal surrounding tissue in order to prosper.
“It’s not yet clear why this happens, it could be to resist treatment. We will be examining mitochondrial transfer in two related diseases—neurodegeneration and neurological cancer. Each disease involves the same neural tissues, however the outcomes for cells in each are vastly different,” says Matt.
Over the past few months Matt has been developing a set of tools to study the mitochondria’s movements.
“Mitochondria are normally labelled with fluorescent dyes, but this method is tricky to control and is not accurate,” says Matt.
“I’ve used a genetic approach to develop a new system that will enable us to measure these transfer events with high levels of precision. Sometimes there are thousands of mitochondria and a few have transferred between cells.
“We’re using unique genetic signatures to give us strong, quantifiable results. This system will allow us to see whether injury drives mitochondrial transfer in diseased cells.”
For more information contact Matt Rowe on matt.rowe@vuw.ac.nz.
Health Research Council funding for Victoria University researchers
22 April 2016
Dr Bronwyn Kivell from Victoria’s School of Biological Sciences and Centre fro Biodiscovery is a recipient of an Explorer Grant in the HRC’s 2016 funding round, worth $150,000 over two years.
Explorer Grants are awarded for research that advances ideas considered to be transformative, innovative, exploratory or unconventional, and have potential for major impact.
Dr Kivell, a researcher in physiology and neurobiology, will use her grant to develop new, improved painkillers that don’t cause addiction or become less effective over time.
“Chronic pain affects one in six New Zealanders, robbing them of their quality of life. It is poorly treated with current medications, which become ineffective with long-term use and have high abuse potential,” says Dr Kivell.
“Our project is focused on a novel chemical called Salvinorin A. With considerable therapeutic benefits over traditional morphine-like compounds, Salvinorin A painkillers promise to transform the treatment of chronic pain, while the potential social and economic benefits of developing such a therapy are enormous.”
Dr Kivell is working with medicinal chemist Professor Thomas Prisinzano from the University of Kansas.
“Our research seeks to develop design parameters for a series of walking paths that measure and track the progress and regress of the physically disabled. We hope to create safe, functional, therapeutic outdoor spaces for people with disability, which they could use in local parks for free.”
“The Explorer Grants are a critical way of allowing researchers to develop new avenues of research that have the potential to change people's lives.
“Victoria researchers are committed to ensuring their work will deliver impact to enhancing patient health and wellbeing”.
Nine Explorer Grants were awarded in 2016, worth a combined total of $1.35 million.
For more information contact Bronwyn Kivell at bronwyn.kivell@vuw.ac.nz.
Biotechnology researcher awarded grant to engineer enzymes
19 February 2016
Associate Professor David Ackerley from the School of Biological Sciences has been awarded a grant worth US$1.5 million to develop enzymes that will enable the study of degenerative diseases in zebrafish.
The National Institutes of Health, a biomedical research facility in the United States, awarded the grant to Associate Professor Ackerley and his research partner Jeffrey Mumm, Associate Professor of Ophthalmology at the Johns Hopkins Wilmer Eye Institute and McKusick-Nathans Institute of Genetic Medicine at the Johns Hopkins University School of Medicine.
“Firstly, my team at Victoria will engineer some enzymes so that they become extremely efficient at activating certain drugs from a non-toxic to a highly toxic form,” says Associate Professor Ackerley. “If these enzymes are present in a living cell, that cell will become highly sensitive to the drugs and die when it encounters them. Importantly, ordinary cells—even those right next to the sensitive cells— will be unaffected.”
The biotechnology researcher will then provide Associate Professor Mumm with a piece of DNA that acts as a blueprint for the engineered enzymes, enabling them to be targeted to any cell type that is desired.
“The original concept for this system was invented by Dr Mumm. It worked fairly well, however our improved enzymes will enable him to far more effectively target certain types of cells in zebrafish, to make those cells sensitive to the drugs,” says Associate Professor Ackerley. “For example, if the enzymes are made only in photoreceptor cells in the retina, application of the drugs will then mimic the effects of degenerative blindness in the zebrafish.”
The processes underlying regeneration or repair of the photoreceptor cells can then be studied, says Associate Professor Ackerley.
“We can screen for new drug candidates that enhance these repair processes. Moreover, the same enzyme tools could be used in a very similar manner in other organs to model other degenerative diseases and to investigate potential cures.”
Tiny enzymes play big role in anti-cancer research
11 January 2016
A $15,000 scholarship awarded to Victoria University of Wellington student Abigail Sharrock will support her quest to develop a new form of cancer treatment.
Abigail, a PhD student in Victoria’s School of Biological Sciences, is one of four students around New Zealand awarded a 2015 Earle Scholarship in Technology.
Administered by Universities New Zealand, the scholarships support postgraduate research into aspects of innovation and product development or bioprocess technology.
Abigail’s research will focus on understanding how specific enzymes—bacterial nitroreductase enzymes—can be used to develop new cancer therapies.
“These enzymes are proving an important tool in the development of new cancer therapies, as they can convert a non-toxic ‘prodrug’—a medication that is inactive until metabolised by the body—to a toxic drug that causes cell death,” says Abigail.
“We want to use this property to develop a cancer gene therapy, in which tumour-specific bacteria will deliver genes that instruct an enzyme to specifically kill cancerous cells.
“The research focuses on developing a treatment with minimal damage to healthy tissues. We will be able to confidently see if the enzymes are confined to the tumour—an important safety feature.”
Abigail says nitroreductase enzymes also contribute to understanding how different tissues and cells regenerate.
“Cell ablation therapy can be used to knock out certain cell types that will in turn mimic a diseased tissue state. This means we can look into how this disease state can be reversed or treated by testing compounds that promote cell regeneration.”
Abigail’s research is co-supervised by Associate Professor David Ackerley at Victoria and Professor Vic Arcus at the University of Waikato, and carried out in collaboration with researchers at John Hopkins University in the United States and the universities of Waikato and Auckland.
Conducting research that has a real-world, medical application has always been her goal, says Abigail.
“Biotechnology is an exciting and rapidly advancing field, and I’m excited to be working on this collaborative project that ultimately aims to improve the health and wellbeing of patients worldwide.”
Ferrier’s focus on the future
4 December 2015

Three different projects focused on solving a variety of problems, which are being led by scientists at Victoria University of Wellington’s Ferrier Research Institute, have been awarded a total of $3 million in Government funding to develop their commercial potential.
The Ferrier Research Institute specialises in carbohydrate chemistry and is focused on bringing better drugs, materials and other technology to the world.
The scientists, Professor Gary Evans, Dr Phillip Rendle and Professor Bradley Williams, are each receiving $1 million over two years from the Ministry of Business, Innovation and Employment (MBIE) through its Smart Ideas science investment round for 2015.
“New Zealand now has an incredibly competitive science funding system, with the smallest approval rates for proposals in the world. The major success by our Ferrier scientists and their research collaborators in this round speaks to the potential impact of the proposed projects and the quality of the ideas.”
Hope for Huntington’s
Dr Phillip Rendle’s research aims to produce a safe and effective treatment for some or all of the nine polyglutamine diseases, the most well-known of which is Huntington’s. These diseases are genetically inherited and rare, affecting around 1 in 10,000 people worldwide.
Dr Rendle says there is currently no cure for these diseases, just treatments for the symptoms, which typically emerge in mid-life and get progressively worse with age.
“I want to find a treatment using dendrimers, which are molecules that look like a heavily branched tree. Polyglutamine diseases are neurodegenerative diseases caused by the abnormal interaction of inherited mutated proteins. Our aim is to use dendrimers to disrupt this interaction to delay the onset age, which would effectively be a cure.”
Dr Rendle says he’ll be working with Professor Russell Snell from the University of Auckland, an expert in Huntington’s disease who will conduct biological testing of the materials being developed in the project.
Reducing infections from surgical implants
Professor Gary Evans is developing materials that will reduce infections which sometimes require orthopaedic implants—such as hip or other joint replacements—to be removed.
Biofilms are produced by microorganisms and form a protective layer that adheres to surfaces. In the case of orthopaedic implants, the majority of post-surgical infections are caused by bacteria growing within a biofilm, which develops on the implant. Where a biofilm is allowed to form, these bacteria are protected from the patient’s immune system and antibiotic treatments.
Professor Evans says he and his team (which includes researchers from Callaghan Innovation and University of Otago) will try to engineer entirely new materials through coating titanium, which is used in the majority of modern orthopaedic implants, with molecules that stop biofilm formation.
“While removal of a joint is only necessary in 1-2% of cases, when that’s applied to 3.7 million such surgeries a year in the United States alone, preventing those infections would have a big impact and save a lot of money,” he says. “As the population ages and expects to be more mobile with the help of joint replacements, the issue is only going to become more pronounced.”
Smoother sailing?
A new kind of paint that would stop barnacles and other organisms from accumulating on the hulls of marine vessels has the potential to revolutionise the shipping industry, according to Professor Bradley Williams.
“The growth of seaweed, barnacles and the like on ships is a major problem for marine industries. It creates lots of resistance, which means slower ships and requires as much as 30% more fuel to maintain shipping speeds for on-time delivery—that’s hard on both the financial bottom line and the environment. The ships need to be dry-docked while the encrustation is removed, which takes time and money. And if a ship is wrecked, the biocides which are currently used in paints to ward off encrustation end up leaching into the ecosystem.
“Mine is an extremely simple idea that brings together existing scientific principles in a new way. But the way this idea intends to solve the problem and the fact it won’t have any impact on the environment means it has the potential to be a game changer for paint manufacturers in the shipping industry.”
The formula for teaching success
10 November 2015
"Every student has potential. The secret is to try to reveal the student's aptitude to themselves, to give them the confidence to help maximise that potential."

That’s the teaching philosophy of Dr Robert Keyzers, a Senior Lecturer in chemistry from the School of Chemical and Physical Sciences and a recipient of a Victoria University Teaching Excellence Award.
The awards recognise a consistently high level of teaching, reflected in both peer and student feedback, as well as ongoing innovation and leadership. "Keeping in mind the specific needs, and the background knowledge and understanding of students are key aspects to helping them on their academic journey. I try to do this by walking down the learning path with them.
“This is made easier by the fact that the subjects I teach are not necessarily 100 per cent connected to my own research. This can help with teaching because, without the complete background, I don’t necessarily know what the students don’t know at the beginning of a semester. As such, the process of discovery means I can look at the material with a similar viewpoint to them,” says Rob.
According to Rob, one of most rewarding experiences in teaching is seeing the “penny drop” with a student who has been struggling with a difficult concept.
“You can really see a gleam in their eyes as it all just clicks into place, which is a magical moment for any teacher.” Rob initially had a goal of becoming a forensic scientist. However, the realisation that he would face a limited job market in this field led him to eventually find a passion for the chemistry of nature.
His research includes bio-prospecting from marine organisms around the Pacific to identify new pharmaceutical compounds for medical applications. He also studies the compounds important to the sensory properties of wine to explore how they can be optimised.
He spends much of his time teaching for programmes, such as biology and geology, but also strives to instil a love of chemistry in students as well.
Rob says the award is a good reminder of the importance of teaching at a tertiary level.
“Teaching is the central core of what a university is about, but I think we, as academics, can often get distracted by the excitement of our research. Awards like this remind us of the importance of nurturing the younger generation of future academics and professionals as they set out on their career path.”
Copying tuberculosis could yield vaccines against malaria and cancer
5 August 2015

Future vaccines might one day contain a tiny whisker of tuberculosis, to boost their power.
Victoria University chemists Bridget Stocker and Mattie Timmer's research into tuberculosis may eventually form part of a vaccine for malaria, one of the biggest challenges for medical researchers today.
One of the difficulties they face is in hitting the right note between the body overreacting or underreacting, Timmer said. "Too much can be dangerous."
But underreacting to a vaccine means the body's immune system "memory" of the disease will be weaker, so immunity will not last as long. Something added to a vaccination that will make the body sit up and take notice – and keenly remember – would be a valuable tool for health researchers.
The Marsden-funded pair believed a chemical, found on the little-studied outer surface of the tuberculosis bacterium, might be just such a tool, Timmer said.
People are born with their immune systems primed to recognise this surface and fight the disease – once the leading cause of death in the Western world, infecting the lungs, lymph nodes, bones, joints and kidneys.
"We can find out what those [chemical] structures are, and then make them, and make modifications to see if we can improve the activity."
Stocker and Timmer would spend about three years creating and testing sister chemicals of this tuberculosis molecule. Their aim was strike a balance between something that effectively activated the immune system but was also simple to make, he said.
The final product would be inspired by the tuberculosis chemistry, but made from scratch in the lab before being included in the vaccine, Stocker said. "It might freak people out, but all we're doing is looking at the molecules on the outside of the bacteria."
The chemical has none of the infection-causing parts of tuberculosis, so no one would be at risk of developing the disease should it be included in a vaccine, Timmer said. "The vaccine can be against anything: pathogens and bacteria and anti-cancer vaccines."
Through her work, Stocker had seen the fears that small numbers of people had about the safety of vaccines. While all would be subject to thorough, independent safety tests, she wondered if a product derived from a "natural" source might be accepted more easily by a chemical-averse public.
"People assume because it's natural it's better. But it's just a perception."
One of the first uses of the research might be in a planned vaccine for malaria, Stocker said. "It was why I got into science – to be able to contribute something."
- Stuff
Ferrier science embedded in biotech drug development
28 July 2015
A new drug developed to treat a debilitating rare muscle-wasting disease and various kidney diseases, and manufactured using Ferrier Research Institute technology, has been acquired by an American company for commercial development.
New Zealand Pharmaceuticals Limited (NZP) announced last week that the Patent License and related Agreements for DEX-M74 has transferred to Altamira Bio, a subsidiary of NASDAQ-listed Fortress Biotech, who will run the clinical development and commercialisation.
DEX-M74, a natural carbohydrate, is currently in a Phase 2 clinical trial for treatment of the rare genetic disorder GNE myopathy, and a Phase 1 trial in patients with kidney disease is scheduled to begin in September.
GNE myopathy, formerly known as Hereditary Inclusion Body Myopathy, is a severe debilitating muscle wasting disease afflicting approximately 2,000 people worldwide. Most patients develop weakened arm, hand and leg muscles in their early 20s and eventually require a wheelchair.
“This is a significant milestone. NZP manufactures DEX-M74 using technology developed and licensed from Ferrier”, says Director of the Ferrier Research Institute Professor Richard Furneaux.
“In fact, the late Professor Robin Ferrier, after whom our Institute is named, was instrumental in developing the manufacturing process when he worked with us in his retirement.”
DEX-M74 has the potential to supplement the patient’s genetic insufficiency by restoring the sialic acid content of their diseased tissues to natural levels.
“Low sialic acid levels are also a feature of many major kidney diseases, so there is cautious optimism that it will have broader applications”, says Professor Furneaux.
Professor Furneaux says Palmerston North-based NZP is going from strength to strength. “We have had a long and productive relationship with NZP. Their Business Development Manager Dr Selwyn Yorke, who has championed the development of DEX-M74, was member of our research team at one point.
Marine sponge shows tumour-stunting promise
7 July 2015

A chemical agent found in marine life unique to New Zealand may hold the secret to fighting certain cancers, according to research co-authored by Victoria University of Wellington’s Professor John Miller and associate professor Peter Northcote.
The research, which has been published in the highly-regarded journal Molecular Cancer Therapeutics, suggests that peloruside A—a substance produced by the marine sponge Mycale henscheli, found mostly in Pelorus Sound—has promising tumour-inhibiting properties when compared to other plant and bacterial-based agents currently used in chemotherapy.
One preclinical trial on lung cancer cells showed tumour growth inhibition greater than 90 per cent with peloruside A, compared with results of 53 per cent and 19 per cent for two current anti-cancer drugs.
A similar preclinical trial on cells of a different type of lung cancer also produced encouraging results, with inhibitions of tumour growth ranging between 50 to 74 per cent, compared to 44 and 50 per cent with the alternatives.
Tests were also conducted on breast cancer cells, with the results suggesting better toleration of peloruside A than the clinically used drugs.
“Although additional research is required, the preclinical results certainly suggest that peloruside A is highly effective in preventing the growth of lung and breast tumours,” says Professor Miller.
“In some cases, there was even a decrease in tumour volume.”
The research also indicates that peloruside A may provide an answer to the growing problem of the acquired resistance of some tumours to current medications.
“This is encouraging, because it means peloruside A could increase the range of options available for long-term treatments; particularly if there are fewer side effects with peloruside A compared with drugs currently used to treat cancer,” says Professor Miller.
Professor Miller believes the results give strong support for further trials. However, advancing clinical studies is challenged due, in part, to a limited supply of the marine sponge.
Efforts are underway to provide enough material, either from aquaculture or large-scale chemical synthesis, to commence human trials.
The research was conducted in association with colleagues from the University of Texas Southwestern Medical Center, Reata Pharmaceuticals, and the CTRC Institute for Drug Development.
Funding to research cancer treatment
7 July 2015
A Victoria University of Wellington PhD student has received a 2015 Todd Foundation Award for Excellence.
Abigail Sharrock from the Centre for Biodiscovery and School of Biological Sciences has been awarded $5,000 for her research project which seeks to understand how tiny biological machines called nitroreductase enzymes can activate anti-cancer drugs for new cancer therapies.
Abigail aims to develop an improved treatment technology with fewer side-effects to provide cancer sufferers with a better quality of life and a much better prognosis. Her research will also have applications in regenerative cell biology studies. Her PhD is supervised by Dr David Ackerley.
Among the other award winners around the country include research projects on alleviating pressure on stormwater networks, improving the performance of seismic energy dissipation mechanisms during earthquakes and developing a real-time cardiac MRI tracking technique.
Applications for the 2016 Todd Foundation Awards for Excellence close on 1 March next year.
E-cigs with nicotine could help women quit smoking - NZ research
16 June 2015

Electronic cigarettes with nicotine could be a useful tool in helping women, in particular, quit or reduce tobacco smoking, New Zealand research suggests.
But the required nicotine-containing liquid was not legally for sale in this country, although electronic cigarettes were, said study co-author Professor Randolph Grace from the Canterbury University Psychology Department.
Some people were ordering the nicotine e-cigs online from a website in China, but many economically disadvantaged people were unable to do so because they did not have credit cards.
The lack of a credit card particularly affected Maori and Pasifika, who were about twice as likely to smoke as other groups, Grace said.
The researchers provided 357 New Zealand smokers who had no intention to quit with a sample of a nicotine e-cig during interviews in November and December 2012.
Overall, participants rated the nicotine e-cigs to be 83.3 per cent as satisfying as their own-brand tobacco, but for women the figure was 91 per cent, while for men it was 74 per cent.
There were 227 participants who agreed to be re-interviewed in February and March 2013, following a 10 per cent rise in tobacco tax. Of that group, 37.8 per cent said they had cut back or made a change in their smoking habit, while 7 per cent had quit.
Most of the reasons given by participants for smoking less were economic, but those who had cut their smoking were also those who had a more favourable impression of the nicotine e-cigs, Grace said.
Perhaps those wanting to quit or cutback were more likely to see the e-cigs as an attractive potentially healthier alternative.
One reason the nicotine e-cigs could be particularly useful for women was that nicotine replacement therapies, such as patches or gum, were less effective in helping women reduce tobacco smoking than men.
"It seems as if the physiological aspect of nicotine addiction is relatively more important in maintaining smoking for men. For women there's relatively more importance for the contextual factors, the behaviour," Grace said.
With e-cigs, people could continue to take part in the behaviour of smoking.
Very strong evidence indicated e-cigs were less harmful than tobacco. The carcinogens in smoking did not come from nicotine but from the burning of tobacco and additives.
Grace said some researchers, including himself, felt a harm minimisation strategy would allow smokers to legally buy nicotine e-cigs in this country if they wanted to quit smoking.
Other researchers, including some practitioners in the addiction field, were against the legalisation of nicotine e-cigs because of concerns young people could become hooked on them.
In his view nicotine-containing electronic cigarettes should be legal for sale to smokers who wanted to use them to quit or reduce the amount of tobacco cigarettes they were smoking, Grace said.
"I wouldn't legalise them open slather because there is a potential for young people to get addicted to nicotine."
Other co-authors of the study were Dr Bronwyn Kivell from the School of Biological Sciences at Victoria University and Dr Murray Laugesen from Canterbury University and Health New Zealand.
Victoria researcher wins funding for revolutionary research
5 June 2015

A Victoria University of Wellington biology researcher has been awarded over $1 million dollars in funding for a revolutionary research project that will “rewrite the textbooks” and could change the way we treat cellular diseases such as brain cancer and Alzheimer’s.
Dr Melanie McConnell says she was nearly speechless when Health Research Council of New Zealand announced it will provide $1,036,746 to fund her three-year project.
“It is very, very exciting. It secures funding to get a team of people working on my research, and allows them to put their heads down and get on with it. Without the grant, the project wouldn’t happen,” she says.
Dr McConnell says the project is based on a discovery made five years ago during her time at the Malaghan Institute of Medical Research, which is based at Victoria University, and was further developed during her current post at Victoria.
The project centres on the discovery that mitochondria can move between cells.
“It’s a new observation that goes against all the dogma in the textbooks. At first, people refused to accept our data. We’ve always assumed mitochondria have to renew themselves within the cell, but the research conducted at Malaghan with Professor Mike Berridge shows that mitochondria can transfer between cells.
“This is potentially a double-edged sword. Cells that are injured in neurodegenerative diseases could use mitochondrial transfer to survive, but cancer cells could also use this process to resist treatment,” she says.
The outcome of her research could change how we treat neurodegenerative diseases such as Alzheimer’s, Parkinson’s and motor neurone disease, where injured brain cells die, and also brain cancers where injured cells are actively growing and resist attempts to kill them.
Dr McConnell will lead the project’s team of five throughout the three-year research period.
“This is only the first step of what could be a 15-year project. Our ultimate goal is to hack the
body’s mitochondrial transfer system to alter cell survival in disease.”
For more information contact Jolene Williams on 04-463 6385 or jolene.williams@vuw.ac.nz
New company to advance potential treatment for cancer and other diseases
21 May 2015

Equity investment for the company, called Avalia Immunotherapies, is coming from New Zealand investment firm Powerhouse Ventures, the New Zealand Venture Investment Fund, Malcorp Biodiscoveries Limited and Victoria Link Limited (Victoria University’s commercialisation office). Additional support is also coming from Callaghan Innovation’s technology incubator programme, in the form of a repayable grant, and the Kiwi Innovation Network.
The director of the Ferrier Research Institute, Professor Richard Furneaux, says Avalia Immunotherapies will further develop the ground-breaking technology and aims to progress it to clinical trials.
The research has been led by Dr Gavin Painter from Ferrier Research and Dr Ian Hermans from the Malaghan Institute, and works as a therapeutic vaccine, activating a patient’s own immune system to recognise and attack cancer cells.
Avalia Immunotherapy’s chief executive, Dr Shivali Gulab, says the decade-long research partnership between Dr Hermans and Dr Painter has led to a powerful technology platform that has
been patented and licensed to the company for commercial development.
“The technology can be used to design new treatments for cancer, as well as infectious disease and allergy. Our initial focus will centre on cancer immunotherapy.”
Professor Furneaux says the potential benefits of the therapy are huge, not only for cancer patients but for the Wellington research community. “I’ve worked in this field since 1980 and this is the first time I’ve been involved in placing our intellectual property in a New Zealand start-up company—that’s how important this research is.
“This is also the beginning of what we hope is a birth of a biomedical initiative for the Wellington region—there’s fantastic biomedical infrastructure here, from research facilities to the excellent District Health Boards. We’re hoping Wellington will become just as well known for its biomedical research as it is for its film industry.”
World first discovery
30 April 2015
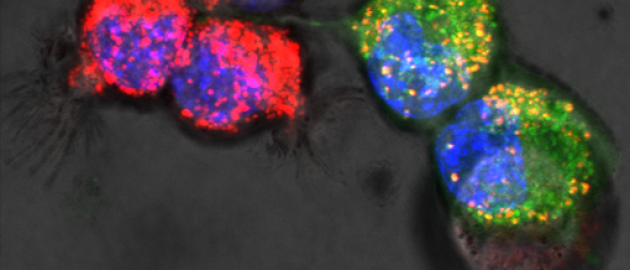
In the first week of January, Malaghan Cancer Cell Biology Group Leader and Centre for Biodiscovery member Mike Berridge and his team, working on breast and melanoma cancer models, discovered that DNA moves from surrounding normal cells to tumour cells with defective mitochondrial DNA.
Senior Research Officer Carole Grasso’s beautiful images highlighting the transfer of fluorescent mitochondria across nanotube membrane connections between cells have been reproduced internationally.
This research opens new possibilities for controlling tumour growth and spread throughout the body. The research could lead to new approaches to treat cancer; to encourage mitochondrial transfer to tumour cells with damaged mitochondrial DNA, and to encourage mitochondrial respiration which discourages tumour growth. Like many discoveries scientists make while investigating one disease, there are implications for another; it may be that this new understanding also offers future treatments for neuromuscular and neurodegenerative diseases involving the sensory organs, and even ageing.
Mike has just visited his co-leader Professor Jiri Neuzil of Griffith University in Queensland and says their publication has opened several new opportunities for collaboration with scientists at the Pasteur Institute in Paris, Virginia Commonwealth University in the USA, the Garvan Institute in Sydney and with others closer to home.
New approaches to breast cancer
30 April 2015

Cancer research at the Malaghan Institute operates on several fronts with many intersecting approaches seeking breakthroughs and moving us closer to new treatments or cures thanks to the committed support we receive.
PhD student Connie Gilfillan is investigating Doxorubicin - a chemotherapy which has been used in the treatment of breast cancer for over 30 years - and investigating the role of dendritic cells in tumours.
“In 1984 Doxorubicin was approved, and while it’s an effective treatment, it shares the fate of many cancer drugs; tumours become resistant to them. I wanted to find out whether the treatment leaves carcinoma cells more immune suppressive after ‘chemo’, or less able to respond to the immune system.”
Immunotherapy hopes to unlock the body’s own fight against cancer; but because cancer cells have incredible dexterity in eluding or hiding from the immune system, they are usually not detected and destroyed. If they are even less likely to be targeted after ‘chemo’ we need to find out additional ways to wake up the immune system.
“Dendritic cells, DCs, are the communicators of the immune system. They are a kind of first alert cell which notices something amiss and then travels back to the lymph nodes to get specialist cells to help. There are many ways that I am testing and probing their role. For example if I take them out of the system completely what happens? Or, if a tumour is immune suppressant does the presence of DCs make things better or worse?” says Connie. “While there is genuine excitement about what we are learning about the various immune cells, there are easily over a hundred to investigate and every small thing discovered adds to the big picture. I look at it like a family tree; the B Cells, the T cells and DCs are at the head of the family but they have a whole family of cells under each, branching out in complexity. I am only looking at one cell and that may occupy me for three years! It is an amazing area of research and the Malaghan Institute is an incredibly stimulating place to be.”
Connie is one and a half years into her PhD under supervisors Professor Franca Ronchese, Dr Melanie McConnell (Centre for Biodiscovery), and Professor Brett Delahunt (Otago).
Hookworm: The Great infection of Mankind, discovery at Malaghan Institute
27 April 2015
In 1962, Norman Stoll, the distinguished Rockefeller Institute scientist who helped to establish human parasitology research in North America, described Hookworm as the Great Infection of Mankind.
Professor Graham Le Gros has led a team which has stimulated both innate and memory responses to the parasite, discovering along the way the unexpected behaviour of one particular immune cell, in Hookworm, one of the world’s most devastating tropical diseases affecting 1 billion[1] people. The journal, Nature Communications, has today published the research [2] .
Ten years of work has seen the Wellington-based scientists demonstrate the interaction between innate and adaptive immune cells, never seen before, providing a credible platform for researchers to work towards preventing a disease that causes global suffering.
Hookworm affects mostly the poorest billion people in the world and, according to the World Health Organisation, contributes to the cycle of poverty and ill health for communities of people living on less than $2 a day. Hookworm infection causes childhood and maternal anaemia, wasting, pain, disability and impaired brain development, but has proved impossible to eradicate as rates of reinfection are high.
Hookworms reproduce in the gut and the eggs passed in stools. The cycle of reinfection continues in poor communities where the lack of both sanitation and footwear makes eradication impossible. The most feasible way to break this cycle would be to create a vaccine for protective immunity but before we can start developing a vaccine against the parasite however, we first need to identify the immune mechanisms that can best protect against hookworm infection.
Graham Le Gros explains, “Humans have evolved to develop immunity to many parasites, but not Hookworm. An unusual feature of their life cycle is that it includes migration of the larvae to the lungs before they develop into adults in the gut.
We were able to create an immune response in the lungs of mice that made it hard for the parasite to live – and therefore break the lifecycle. Our hunt is now to find the right hookworm protein to combine with an adjuvant triggering the activation of these immune cells - to teach a human body to have a memory of how to fight Hookworm.”
Additionally, the team demonstrated one immune cell - type 2 innate lymphoid cells ( ILC2s) - trigger immunity and work with other immune cells, the Macrophages and T helper cells. Previously ILC2s were not thought to be involved as an effector cell in long term memory response to fight off the disease.
The research was assisted by synthetic chemist Dr Gavin Painter from the Ferrier Research Institute and Centre for Biodiscovery at Victoria University of Wellington. The Ferrier Institute are regular collaborators with scientists from the Malaghan Institute. The work was funded by the Health Research Council of New Zealand.
The Centre for Biodiscovery and the Malaghan Institute of Medical Research present Dr Catherine Drummond
Department of Oncology, St Jude Children’s Research Hospital, Memphis
Finding new targets for cancer therapy
Wild-type p53 is expressed by approximately 50% of all tumours at diagnosis and is often accompanied by aberrations in upstream signalling pathways. Reactivating p53 is an attractive treatment therapeutic strategy in these tumours and disrupting the interaction between p53 and its negative regulator MDM2 has been a particular focus. However, as with other targeted therapies, resistance and relapse following treatment is likely. There is a need to both understand how resistance to small molecule activators of p53 might develop in a clinical setting as well as identify alternative ways in which these tumours can be selectively targeted.
Research conducted in the Lunec Lab (Northern Institute for Cancer Research, UK) was aimed at understanding how resistance to MDM2/p53 binding antagonists might arise. Two independent cell lines with resistance to chemically distinct classes of antagonists were generated and following detailed characterisation found to have identical TP53 mutations. These mutations were found to be present at low frequency in the parental populations, suggesting that these mutations were selected for during exposure to low concentrations of the antagonists. However, as these cell lines remained sensitive to ionising radiation these results also suggest that in a clinical setting, patients might respond to conventional chemotherapy.
Identifying alternative ways of targetting tumours expressing wildtype p53 was the the focus of work conducted in the Lain Lab (Karolinksa Institutet, Sweden). 20,000 small molecules were screened in a cell based p53 activation assay in order to identify bioactive compounds. MJ05 was one of the top hits identified in this screen, and was found to have cytotoxic effects in ARN8 melanoma cells (sub-G1 fraction of 16%) whilst having little effect on human normal dermal fibroblasts. MJ05 was subsequently found to be inhibiting S phase progression and, unlike other inhibitors of S-phase, did not activate or inhibit DNA damage response pathways. Whilst these effects of MJ05 were p53 independent, MJ05 also selectevly increased the cytotoxicity of the MDM2/p53 binding antagonist nutlin-3 (from 1.5% to 64%), suggesting utility in both p53 wildtype and mutant tumours.
More recently, studies in the Hatley Lab (St Jude Childrens Research Hospital, US) have been focussed specifically on a transgenic mouse model of embryonal rhabdomyosarcoma (ERMS). ERMS is a soft tissue malignancy that, despite being predominantly p53 wildtype, is observed clinically to express a diverse range of mutations. Furthermore, the origin of these tumours is unknown making the development of targeted therapeutics difficult. Lineage tracing studies in this mouse model are currently in progress, and whilst still ongoing highlight the potential for target discovery in transgenic models.
Tuesday 5 May 2015, 12pm - HMLT104
Chemists make headway in Alzheimer's research

Victoria University of Wellington researchers have made a significant step forward in the search for a treatment for Alzheimer's disease.
Chemists at the Ferrier Research Institute have discovered a way to create cluster compounds for controlling the process that leads to generation of amlyloid plaques in the brain disease.
In 2013, the chemists synthesised a type of complex sugar for the same purpose, in challenging 55 step synthesis. The new approach to construct single-entity clusters reduces the number of reaction steps by half.
“We wanted to simplify the synthesis without losing the desired potency, which is quite challenging,” says project leader Dr Olga Zubkova. “The new products will be easier and cheaper to make, and allow us to prepare larger amounts for various testing.”
Through the joint project, Ferrier chemists Dr Zubkova and Professor Peter Tyler worked with Professor Jeremy Turnbull and Dr Scott Guimond from University of Liverpool to construct new heparan sulfate glycomimetics with critical functions that control the activity of the beta-secretase enzyme in the brain. This enzyme catalyses the first step in the generation of amyloid plaques in Alzheimer’s Disease.
“We designed and decorated a more simplified dendritic core by using multiple short, more readily synthesised fragments”, says Dr Zubkova. “Though we significantly simplified the structures we still saw impressive amounts of bioactivity.”
Dr Zubkova says this provides a highly desirable product that could be used in the development of new treatments.
“These molecules involve sophisticated chemistry processes and target enzymes extremely well, which is crucial for pharmaceutical application. It could be used in numerous treatments as the role of heparan sulfate becomes better understood.”
The research, published recently in prestigious international journal Angewandte Chemie, was supported by funding from the Ministry of Business, Innovation and Employment.
The team has prepared a large collection of the compounds, with approximately 80 new intermediates as well as 11 final products as pure single molecules, something not previously achieved by any research group in the world.
“Others have tried by attaching highly charged fragments to the core which leads to a very complex mixture”, says Dr Zubkova. “But we’ve done it differently—we attach the fragments first before we sulfate them. We have, for the first time, prepared single-entity compounds presenting polyvalent display of heparan sulfate saccharides. That’s a point of difference and what makes our compounds unique.”
The Centre for Biodiscovery, the Malaghan Institute of Medical Research and the Maurice Wilkins Centre for Biodiscovery present Dr Jill O'Donnell-Tormey, Ph.D.
 Chief Executive Officer and Director of Scientific Affairs
Chief Executive Officer and Director of Scientific Affairs
Cancer Research Institute, New York
Cancer Immunotherapy: A Not-For-Profit Vantage Point
Cancer immunotherapy, a class of treatment that harnesses the immune system's power to target and eliminate cancer, has recently enjoyed a renaissance of interest thanks to significant clinical successes. Several active immunotherapies have received FDA approval, and many more are making their way through the clinical development pipeline. The Cancer Research Institute (CRI), a U.S.-based charity that is dedicated exclusively to advancing immunotherapy for all cancers, is playing a critical role in the discovery, development, and optimization of cancer immunotherapies, especially combination approaches that bring multiple drugs together to achieve maximum clinical benefit. In this talk, we will review CRI's model for philanthropic innovation and discuss how it may provide a roadmap for rational and efficient development of immunotherapy across industry and academia.
Jill O’Donnell-Tormey, Ph.D., is Chief Executive Officer, and Director of scientific affairs of the Cancer Research Institute (CRI), a nonprofit organization founded in 1953 that is today the global leader in supporting and coordinating research aimed at harnessing the immune system’s power to conquer all cancers. She joined the organization in 1987 as director of scientific affairs, and has been chief executive since 1993.
Prior to joining CRI, she served as a research associate in the department of medicine at Cornell University Medical College and as a postdoctoral fellow in the laboratory of cellular physiology and immunology at The Rockefeller University. She holds a Bachelor of Science degree in chemistry, summa cum laude, from Fairleigh Dickinson University, and a doctor of philosophy in cell biology from The State University of New York’s Downstate Medical Center.
For more information about the Cancer Research Institute, please click here
Refreshments will follow the lecture
All interested parties are welcome to attend
Please RSVP to Charlotte Ansell at charlotte.ansell@vuw.ac.nz by Friday 27 February
2015 Ferrier Lecture
 The 2015 Ferrier Lecture will be given by Professor Larry Overman from the University of California, Irvine at Government Buildings, Wellington, onTuesday 3 March.
The 2015 Ferrier Lecture will be given by Professor Larry Overman from the University of California, Irvine at Government Buildings, Wellington, onTuesday 3 March.
Time 5:30 pm refreshments, 6:00 pm lecture
Venue Lecture Theatre 1, Government Buildings, Stout St
RSVP with this form by 25 February please.
Professor Overman will also give lectures to the NZIC Otago Branch in Dunedin on Wednesday 4 March and the NZIC Auckland Branch on Monday 9 March. Times and venues for these events will be advised in due course.
Title and abstract
Natural Products Synthesis: Insights into Chemical Reactivity and Inspiration for New Antitumour Agents.
Progress in organic chemistry has been closely linked to natural products since Serturner’s isolation of pure morphine in 1803 and Woehler’s conversion of ammonium cyanate to urea in 1828.
This lecture will describe two recent natural products total synthesis projects in the Overman laboratory, one that led to new strategies for coupling complex molecular fragments and another to a new class of preclinical epigenetic antitumor agents.
About the Ferrier Lecture
It was Robin Ferrier’s particular belief that young chemists could benefit greatly from mixing with leaders in their field. Therefore, invited Ferrier Lecturers, who are recognised internationally in chemistry or a related field, are brought to New Zealand to engage with postgraduate students as well as lecture.
The Ferrier Lecture is supported by private donors, including Dr Peppi Prasit (one of Robin’s former PhD students), as well as the New Zealand Institute of Chemistry (NZIC) and the Faculty of Science at Victoria University.
Despite failing health, Robin attended the inaugural Ferrier Lecture, given by Professor Vern Schramm in March 2013, but died later that year.
Kiwis help with lab-grown retina
4 February 2015
In what sounds like a gruesome sci-fi plot, Victoria University researcher David Ackerley is preparing to grow an artificial retina.
The biotechnologist, and others in a world-leading international team, hope it could help to cure one of the most common forms of vision loss.
The retina is a layer of light-sensitive cells at the back of the eye, connecting to the brain and allowing us to see. Though its cells consistently regrow themselves, this is an imperfect process - and some people are genetically predisposed to more frequent damage or the repair going astray, leading to degenerative blindness.
The "retina in a petri dish" will be grown from stem cells at Johns Hopkins University in the United States. Ackerley's team at Victoria and a second at Johns Hopkins will act as chefs, designing the ideal DNA recipe.
Having successfully grown a prototype healthy one, their next step is to make an unhealthy one that mimics degenerative blindness.
"Now you've got it working, the question is how do you make it stop working in a way that mimics degeneration [of vision]," Ackerley said. "The key thing is you want to leave most of the retina intact."
The international team has been given a US$500,000 (NZ$684,000) grant for this work.
The trick to knocking out specific visual cells was to add instructions to their DNA that made them die when exposed to an otherwise-harmless substance, leaving the cells around them unharmed.
Ackerley, with the Auckland Cancer Society Research Centre, is currently studying how transporting such DNA recipes into cancer cells could become a revolutionary new treatment. His team's experience in this made him ideally placed to join the international research.
Once they have grown the new retina, they will begin first by killing cells, then seeing what medicines help the retina to repair itself.
"I wouldn't say we've got a cure for blindness . . . It allows you to look in a way that can't currently be done for new drugs."
The example could be followed for studying diseases in other organs as well.
"This is a great system for just being able to look at the cells without harming any animals, and actually looking at the human response."
ASSASSINS' RECIPE
Bacteria and human cells can have a vastly different reaction to a substance - it's why when taking an antibiotic the drug will kill bacteria without affecting us.
David Ackerley's DNA recipe instructions borrow from the bacteria cookbook, specifically the steps to make a certain enzyme. The original enzyme converts an otherwise-harmless substance into a toxic one, poisonous enough to kill a cell. Ackerley's lab has since turbo-charged it, making the enzyme highly efficient.
By inserting these instructions into the same page of the cookbook as the recipe for a visual cell means just these specific cells will use them.
Therefore, when the harmless substance is added to the artificial retina, the light-receptive cells will make the toxin while the recipe will stay inert and unused in all other cells, which will live.
- The Dominion Post
US funding to research retinal disease
29 January 2015
 Dr David Ackerley, from Victoria University’s Centre for Biodiscovery, is part of a team awarded a US$500,000 Falk Medical Research Trust grant to develop new models of retinal degenerative disease—–a major cause of human blindness.
Dr David Ackerley, from Victoria University’s Centre for Biodiscovery, is part of a team awarded a US$500,000 Falk Medical Research Trust grant to develop new models of retinal degenerative disease—–a major cause of human blindness.
Dr Ackerley will work alongside Dr Val Canto-Soler and Dr Jeff Mumm from the Johns Hopkins University in Maryland, USA, to build an artificial retina of the human eye that mimics degenerative disease.
“My lab group will be developing genetic methods to enable very precise killing of specific cells in the artificial retina to permit study of how they regenerate, and facilitate discovery of drugs that assist with this process,” says Dr Ackerley.
Dr Val Canto-Soler recently developed world-first methods to induce human stem cells to grow into an artificial retina in a Petri dish.
Dr Ackerley has previously collaborated with Dr Mumm to create a system for effectively killing specific living cells without harming surrounding tissues, using specially engineered enzymes.
The Falk Medical Research Trust grants one year of funding to find new cures for diseases or improve existing treatments.
Biodiscovery scholar cleans up prizes
26 January 2015
 Last month, Cameron Field won the Student Poster Prize at the Australasian Society for Immunology’s (ASI) widely attended and stimulating annual scientific meeting. The week-long conference, in New South Wales, was attended by Nobel Laureate Bruce Beutler. Together with Jules A. Hoffmann, he received one-half of the 2011 Nobel Prize in Physiology or Medicine, for ‘their discoveries concerning the activation of innate immunity’. Ralph Steinman was awarded the other half of the Nobel Prize post-humously for his discoveries concerning the activation of adaptive immunity.
Last month, Cameron Field won the Student Poster Prize at the Australasian Society for Immunology’s (ASI) widely attended and stimulating annual scientific meeting. The week-long conference, in New South Wales, was attended by Nobel Laureate Bruce Beutler. Together with Jules A. Hoffmann, he received one-half of the 2011 Nobel Prize in Physiology or Medicine, for ‘their discoveries concerning the activation of innate immunity’. Ralph Steinman was awarded the other half of the Nobel Prize post-humously for his discoveries concerning the activation of adaptive immunity.
Dr Beutler was guest presenter at the conference’s Post-Graduate workshop which challenged attendees to solve their own immunological puzzles; a highly relevant challenge for Cameron and his peers.
Cameron’s poster, part of his PhD project, investigates immunotherapy possibilities for Glioblastoma multiforme (GBM), a rapidly fatal brain cancer, currently with limited treatment options. The poster win came less than a week after he received the runner-up Emerging New Investigator award, presented annually by the Wellington Health and Biomedical Research Society. The term new investigator is not related to chronological age but recognises substantial research endeavours started within the previous five years.
He shares his enthusiasm and determination to further our understanding of GBM, “Many tumours are good at side-lining or suppressing the immune system, and GBM has proved harder than most, but my study involves combining drugs to limit this suppression, with a vaccine.”
“A new class of drugs called ‘checkpoint inhibitors’, recently approved by the US Food and Drug Administration (FDA), are designed to block the inhibitory signals within the immune system that apply the ‘brakes’ to an immune response. When we cut the brake lines, we are able to unleash a more powerful immune response.”
Immunotherapy and cancer have become increasingly used in the public domain since the journal Science named cancer immunotherapy its 2013 Breakthrough of the Year, but a body of academic work, over several decades led to this public ‘tipping point’.
New Zealand and Australia; traditional rivals, joined forces 24 years ago to form The Australasian Society for Immunology, encouraging and supporting the discipline of immunology in our region and introducing young scientists to the discipline. ASI members have been prominent in advancing biological and medical research worldwide.
South Auckland-born Cameron, hopes to complete his PhD this year and join the growing list of New Zealand born scientists making their mark internationally. “By the end of the year I hope to complete my PhD. It’s been fantastic working at the Malaghan Institute for the last three years, under the supervision of Ian Hermans. “Both Ian and the Malaghan Institute command a high standard of research and this bodes well with our presence both nationally and internationally. Being challenged in this manner will more than prepare me for a research career ahead.”
Asthma vaccine discovery
7 October 2014
With asthma now affecting up to one in four New Zealand children, the researchers say this is a promising step in the challenge to understand and control asthma.
The experimental approach is one of the newest frontiers in the rapidly advancing field of immunotherapy, which harnesses the body’s own ability to fight diseases.
The research is an extension of work at the Malaghan Institute of Medical Research developing vaccines for cancer by Associate Professor Ian Hermans, in collaboration with synthetic chemist Dr Gavin Painter from the Ferrier Research Institute at Victoria University of Wellington where the vaccines are designed and synthesized.
"Cancer and asthma both involve the immune system, but in cancer we are trying to get the body to take notice of tumour proteins, while in asthma, we want to stop it over-reacting to an allergen," says Dr Hermans.
"Allergy is the wrong sort of immune response. Using the vaccine, we have initiated a more appropriate immune response and prevented the allergy from taking hold.
"Vaccines work by presenting the body with an antigen, which provokes an immune response. This involves activating T cells, produced by the body's immune system, which are then ready to protect from the disease in the future.
To strengthen the immune response, a chemical called an adjuvant is administered along with the antigen, to make the vaccine more effective.
In the asthma vaccine, the antigen and the adjuvant are chemically linked, rather than simply co-delivered. This novel approach ensures the essential components reached the target cells together and created the most powerful but highly specific immune response that targets the disease.
Dr Herman’s says preparing the linked vaccine required some "pretty clever chemistry".
"By linking them, we make sure they are both delivered to the right place in the body. Once there, they are split and presented to the immune system to initiate a response," he says.
The idea of using a vaccine to prevent asthma was the brainchild of Malaghan Institute Professor Franca Ronchese who explains how the vaccine works.
"In asthma, allergens such as those produced by house dust mites are inhaled and taken up by dendritic cells in airways, causing inflammation and many of the symptoms of asthmatic disease. With the vaccine, we think we can direct other immune cells, the killer T-cells, to go and block the dendritic cells, so they stop sending out the wrong messages. It’s like taking out the generals of the enemy’s army in order to overpower it," she says.
The linked vaccine technology could in principle be applied to other allergic diseases. Patent protection has been obtained and opportunities to commercialise the technology are currently being pursued.
The work was jointly funded the Health Research Council of New Zealand and the Ministry of Business, Innovation and Employment of New Zealand.
The paper can be viewed online here
Quality of undergraduate research recognised
29 September 2014
Research by three Victoria University of Wellington undergraduate students has been published in Biotechnology Letters, a highly ranked international peer-reviewed journal.
 As part of a supervised programme of study, Madeleine Parker, Kate Walmsley and Jack Sissons, each in the final year of a Bachelor of Science majoring in biotechnology, worked to develop an efficient system to help scientists artificially evolve enzymes in the lab.
As part of a supervised programme of study, Madeleine Parker, Kate Walmsley and Jack Sissons, each in the final year of a Bachelor of Science majoring in biotechnology, worked to develop an efficient system to help scientists artificially evolve enzymes in the lab.
“Directed evolution is a method of enzyme improvement in which a gene is randomly mutated and a large number of gene variants are produced,” says Madeleine. “Testing the slightly different enzymes encoded by these variants requires cloning them into bacterial cells and screening for the desired activity.
”Kate adds that many directed evolution studies expend unnecessary effort testing cells that have not received a gene variant at all. “Our system allows us to produce bacteria where 100 percent of cells contain the gene of interest,” she says.
Jack explains that this allows the team to test the activity of each slightly different enzyme in the most efficient manner possible. “Achieving this is of great value because we don't want to waste time and resources screening bacteria that aren't doing what we want them to.
”The original idea for this work was developed by Victoria PhD graduate Dr Gareth Prosser. Jack, Madeleine and Kate performed the key proof-of-concept experiments to validate Dr Prosser’s idea under the supervision of Dr David Ackerley, Biotechnology Programme Director, and Dr Elsie Williams, a postdoctoral fellow in the School of Biological Sciences.
“It is rare for undergraduate research to be published at all, let alone in a well-regarded journal like Biotechnology Letters,” says Dr Ackerley. “Kate, Jack and Madeleine worked hard and intelligently on this project, and really deserve their success.”
The paper can be viewed online here.
Connecting Curiosity - Tales of Science Serendipity - TEDxWellington
Centre for Biodiscovery member Dr Laura Green recently presented at the TEDx conference held in Wellington at the end of August. Her talk centred around how scientists need to be challenged to tell better stories - whilst sharing how potentially the research Laura has been involved with might have found drugs to treat MS.
Recently awarded the 13th Zonta Science Award, Laura has been recognised as an up-and-coming female scientist to watch. She is passionate about making everyday science accessible to everyday people and is currently collaborating with local entrepreneurs to develop entertaining and engaging science media through effective storytelling, humour, and of course, cartoons.
Check out her talk below:
Promising new treatments for multiple sclerosis
15 August 2014
New treatments for multiple sclerosis (MS) using common anti-psychotic agents have been discovered by Victoria University of Wellington researchers.
The study led by Dr Anne La Flamme, an associate professor in the School of Biological Sciences and head of the MS research programme at the Malaghan Institute of Medical Research, based at Victoria, shows the potential of clozapine and risperidone to effectively treat MS.
MS, a neurological disease which affects one in every 1,400 New Zealanders, is caused by immune cells invading the brain and causing inflammation. It leads to impaired vision and coordination and, eventually, paralysis, explains Dr La Flamme.
“While disease-modifying drugs are currently available, they are often effective in only a subpopulation of MS patients and all of these treatments target the disease through traditional immune pathways,” she says.
“What makes our findings so important is that clozapine and risperidone target a very different set of pathways from all other MS drugs, and thus have the potential to treat those MS populations for which no effective therapies currently exist.”
Published this week by international scientific journal PLOS ONE, the study demonstrates that risperidone and clozapine can reduce MS significantly by reducing the inflammation in the brain that causes this disease.
Additionally, this research indicates that the way clozapine and risperidone improve disease outcomes in MS is different from how these agents work to treat mental health disorders.
“By utilising existing therapies, this work may more quickly support improved outcomes for people with MS,” says Dr La Flamme.
This study, funded by the Neurological Foundation of New Zealand, was undertaken in collaboration with Dr Bronwen Connor, an associate professor at the University of Auckland.
The PLOS ONE article can be viewed online here.
For more information contact Dr Anne La Flamme on 04-463 6093, 021 555 413 or email anne.laflamme@vuw.ac.nz.
Using synthetic biology to make new antibiotics
22 July 2014
 Research at Victoria University of Wellington could lead to a new generation of antibiotics, helping tackle the global issue of ‘superbugs’ that are resistant to modern medicine.Led by Mark Calcott, who has just completed his PhD study, under the supervision of Dr David Ackerley, an associate professor in the School of Biological Science, the research is delivering new knowledge about how synthetic biology might be used to counter bacteria that have become resistant to existing antibiotics.
Research at Victoria University of Wellington could lead to a new generation of antibiotics, helping tackle the global issue of ‘superbugs’ that are resistant to modern medicine.Led by Mark Calcott, who has just completed his PhD study, under the supervision of Dr David Ackerley, an associate professor in the School of Biological Science, the research is delivering new knowledge about how synthetic biology might be used to counter bacteria that have become resistant to existing antibiotics.
The recently published study defines new ways that microbes, which are used to make some commonly used types of antibiotics, can be reengineered to produce modified forms of the original molecules.
“Part of the problem is that people have historically been careless when using antibiotics, which has, one-by-one, allowed bacteria to build resistance, thrive and multiply. We’re smarter now, but at a time when we’re running out of options,” says Dr Ackerley.“There is a serious and immediate need for new antibiotics—either we have to develop the next generation or find clever and affordable ways of modifying the ones we currently have,” he says.“The basis of our research is the idea that the microbial machinery (enzymes) that makes a particular antibiotic can be rearranged, to make a different antibiotic that resistant bacteria won’t recognise. The new antibiotics will still fight infection, and if we can use them in a more targeted way, bacteria won’t become resistant so easily.”
He says the ultimate goal of the study is to be able to produce high yields of new and affordable antibiotics that ‘superbugs’ don’t recognise and are not resistant to.
Results have been published by the American Society for Microbiology journal Applied and Environmental Microbiology, viewable online here.
The research is a core output of a grant Dr Ackerley has received from the Marsden Fund for a project called Cracking the non-ribosomal code. Dr Ackerley is collaborating with Professor Iain Lamont from the Department of Biochemistry at Otago University.
For more information contact Dr David Ackerley on 04-463 5576 or email david.ackerley@vuw.ac.nz.
Fighting paediatric disease through research
4 July 2014
A Victoria University of Wellington researcher is one step closer to identifying candidate treatments to delay the onset and progression of a fatal paediatric disease for which no effective therapy currently exists.
Work by Dr Andrew Munkacsi from Victoria’s School of Biological Sciences and Centre for Biodiscovery, in collaboration with Professor Mengjie Zhang from the School of Engineering and Computer Science, and Dr Stephen Sturley from Columbia University, is delivering new knowledge about the rare neurodegenerative disease, Niemann-Pick type C (NPC).
NPC is a monogenic disease caused by a defect in one of two different genes that affects approximately one in 150,000 children worldwide. Those affected are typically born without symptoms, but within a few years exhibit dementia similar to Alzheimer's disease and usually die before reaching adolescence.
Using exome sequencing, a strategy that selectively investigates important sequences of genetic material in all 23,000 human genes, Dr Munkacsi is analysing DNA samples from siblings in Australia, the United Kingdom and United States who have NPC disease to identify underlying disease gene mutation.
"Affected siblings, by inheritance, have the same mutation in the disease gene, but the onset and progression in the cohort we are studying is different. What we hope to do is identify genes associated with disease severity," says Dr Munkacsi. Dr Munkacsi has been researching NPC for the past nine years.
Through his investigations with Dr Sturley, using a yeast model of NPC disease, they have demonstrated that there are genes other than the disease-causing genes that modify disease severity.
This strategy has been successful, and has identified a drug that will be further tested in a human clinical trial in the United States. They are now going to the next level and conducting the first genome-wide analysis of sibling pairs affected with NPC disease.
"Once we identify which genes regulate the onset and progression of NPC disease, we can work towards targeting those genes with drugs. Our goal is to identify drugs already on the market as the children do not have the time to wait for new drugs to be developed and approved.
"As terrible as Alzheimer’s disease is, at least persons affected live a healthy life for 60 to 80 years. Children affected with NPC disease deserve a chance to live a healthy life," says Dr Munkacsi.
For more information, please contact Dr Andrew Munkacsi on Andrew.Munkacsi@vuw.ac.nz
Researcher to give lectures in United Kingdom
26 May 2014
 Dr Bridget Stocker, a senior lecturer in the School of Chemical and Physical Sciences, will present a two-week sponsored lecture tour in the United Kingdom next month.
Dr Bridget Stocker, a senior lecturer in the School of Chemical and Physical Sciences, will present a two-week sponsored lecture tour in the United Kingdom next month.
Dr Stocker received the 2011 Easterfield Medal, given in honour of the late Sir Thomas Hill Easterfield, in recognition of significant research by an emerging chemist. As part of the award, Dr Stocker is being sponsored to visit Oxford, York, Edinburgh, St Andrews and Cambridge Universities to talk about how glycolipids can help in understanding and treating disease.
"This is a tremendous opportunity to not only visit such prestigious universities in the United Kingdom, but also to showcase what we do here in Wellington," says Dr Stocker.
"It's important to get your name out there and to meet like-minded people in your field. The advantage of a trip like this, over say a conference, is that you really get to have more one-on-one time with very intelligent and inspiring people. Enthusiasm is infectious and lends itself to the generation of a lot of new ideas."
Dr Stocker was the top graduating Victoria University Bachelor of Science (Hons) student in 2000. She went on to complete a PhD that focussed on the total synthesis of several anticancer agents. Following a brief period as a lecturer, Dr Stocker was awarded a fellowship to spend two years at the prestigious Swiss Federal Institute of Technology, Zurich.
In 2006, Dr Stocker returned to New Zealand and established an immunoglycomics research programme at the Malaghan Institute in collaboration with partner Dr Mattie Timmer, a senior lecturer in the School of Chemical and Physical Sciences. She is currently a Health Research Council Sir Charles Hercus Research Fellow.
The focus of Dr Stocker’s research involves understanding the role of carbohydrates in immunology. Key highlights include the development of new ‘green chemistry’ methodologies and the synthesis of carbohydrate ‘probes’ to study diseases, such as cancer.
Winner of 2014 Zonta Science Award
7 May 2014
 Dr Laura Green, who is part of a Victoria University of Wellington team researching better ways of treating the debilitating symptoms of multiple sclerosis, has won the 2014 Zonta Women in Science Award.
Dr Laura Green, who is part of a Victoria University of Wellington team researching better ways of treating the debilitating symptoms of multiple sclerosis, has won the 2014 Zonta Women in Science Award.
His Excellency, Lt Gen The Rt Hon Sir Jerry Mateparae, GNZM, QSO, Governor-General of New Zealand presented Dr Green her prize at a special reception hosted at Government House.
The Zonta Science Award provides Dr Green with $15,000 prize money, and $3,000 to be put towards overseas travel. She will use the funding to travel to Switzerland to work with an eminent researcher who has developed a new imaging technique that can visualise individual immune cells trying to gain entry to the central nervous system.
“I will then bring this specialist knowledge back to New Zealand,” says Dr Green who is a Postdoctoral Fellow in Immunology at the Centre for Biodiscovery, School of Biological Sciences. She held her first research position at the University of Wisconsin-Madison in the United States at the age of 17, and has been involved in biomedical research ever since.
In 2003, Dr Green came to New Zealand and has held research positions at Massey University, and the Malaghan Institute of Medical Research based at Victoria.
She obtained her PhD in Cellular and Molecular Biology at Victoria in 2012.
Dame Margaret Sparrow, Convener of the Zonta Science Award, says the judges were impressed not only with Dr Green’s commitment to science but also her community involvement, notably her enjoyment of public speaking and her enthusiasm for competitive road cycling, which includes assisting with cycle safety programmes and cycling skill clinics.
“Laura is passionate about making science accessible to the wider public and is involved in a number of projects including the use of cartoons and film to make science more exciting.”
Guest lecture to focus on new drug developments
25 February 2014
The results of drug studies which could provide new treatments for life threatening infections will be the topic of the annual Ferrier Lecture presented next month by a visiting lecturer to Victoria University of Wellington.
Jef De Brabander, Professor of Biochemistry at The University of Texas (UT) Southwestern Medical Center at Dallas, United States, is delivering the annual Ferrier Lecture, established in honour of Emeritus Professor Robert (Robin) J. Ferrier, one of New Zealand’s eminent chemists and a leader in the field of carbohydrate chemistry.
In his lecture, Professor Brabander will discuss a unique drug development aimed at the treatment of pneumonia and other life threating infections.
He will present the results of his chemical and biological studies related to an antibiotic called Mangrolide A, isolated from a microbe found in the mangrove swamps in the Bahamas. The studies were carried out in collaboration with John MacMillan at UT Southwestern Medical Center.
"The frequency of antibiotic-resistant bacteria is currently rising at an alarming rate, so the need to identify new antibiotics has reached a critical level," says Professor De Brabander.
Supported through the Victoria University Foundation by Dr Peppi Prasit, a former PhD student of Robin Ferrier, the New Zealand Institute of Chemistry, and Victoria’s Faculty of Science, the Ferrier Lecture aims to bring internationally-renowned scientists to Wellington to inspire current students and deliver a public lecture.
New knowledge about treating multiple sclerosis
4 February 2014
 New information that could lead to improved treatment of multiple sclerosis (MS) has been uncovered by Victoria University of Wellington scientists.
New information that could lead to improved treatment of multiple sclerosis (MS) has been uncovered by Victoria University of Wellington scientists.
A study carried out at Victoria, and recently published online in the international scientific journal PLOS ONE, holds promise for patients suffering from secondary progressive MS, an advanced form of the disease, which causes nerve degeneration leading to impaired vision and coordination, and eventually, paralysis.
The study focused on understanding how a new MS drug, MIS416, developed by the New Zealand biotech company Innate Immunotherapeutics, is able to help patients with secondary progressive MS, a form of MS with few effective treatments.
The team of scientists includes Dr Anne La Flamme, an Associate Professor in Victoria’s School of Biological Sciences and head of the MS Research Programme at the Malaghan Institute of Medical Research, PhD student Madeleine White, and Dr Gill Webster from Innate Immunotherapeutics.
“We know this drug works, but we are not sure why. This study has helped us understand the pathways that are driving the disease and how the medication alters the immune system, giving us a better idea of why MIS416 works as well as insight into how to treat patients and predict who will do better on this sort of medication,” says Dr La Flamme.
Most people believe MS revolves around T cells, says Dr La Flamme, but the Victoria study reveals that targeting other cells in the central nervous system can significantly reduce advanced forms of MS.
For more information contact Dr Anne La Flamme on 04-463 6093 or anne.laflamme@vuw.ac.nz. You can read the full article here
